I recently contracted COVID-19 and it took three weeks of healing to feel well again. And, while I had no lingering effects, many other patients are not so fortunate. Long-COVID i.e., lingering signs and symptoms that can last months or even years with debilitating chronic manifestations, are a reality for far too many patients. As such, we still face an ongoing and urgent need for effective prevention and novel treatment options.
In Cannabis and Corona Viral Diseases: A Review of the Science–Part 1 we covered the history of corona and the initially endocannabinoid system-related pre-clinical data published between mid-2020 and January 2021. Results were promising and potential treatment trends were emerging highlighting various cannabis constituents including CBD, THC, and the terpene eucalyptol. Activation of the body’s own endocannabinoid receptors 2 (CB2) has been proposed as a possible target receptor.

Since the first article, several additional experiments have been completed adding more insight and focus on potential treatment trends. Most of the latest trials are still pre-clinical in nature i.e., they have not been tested or confirmed in humans. Until now. The first clinical trial results come to us from Israel where researchers completed the first such trials. The momentum in the clinical setting is accelerating as the international research communities continue to take active steps toward completing several novel clinical trials examining the use of cannabinoid-based therapeutics in the treatment context of COVID-19. According to the ClinicalTrials.gov database numerous clinical trials are currently underway examining individual cannabis constituents such as CBD, or both THC and CBD, their synthetic derivatives, or whole plant-based cannabis products in the prevention and treatment context of corona viral diseases.
The Endocannabinoidome and Corona Viral Diseases: A Mini Review
Part 2 of our ongoing review of the latest scientific literature contains nineteen new trials that directly examined components of the endocannabinoidome (ECD) in the treatment context of corona viral diseases such as COVID-19. The ECD is defined as the larger environment that interacts with the endocannabinoid system (ECS) including cannabinoids, cannabinoid-sensitive receptors (see graphic below), their metabolizing enzymes, the lipidome, the microbiome, and numerous other compounds or food items such as turmeric, echinacea, or plants rich in beta-caryophyllene.
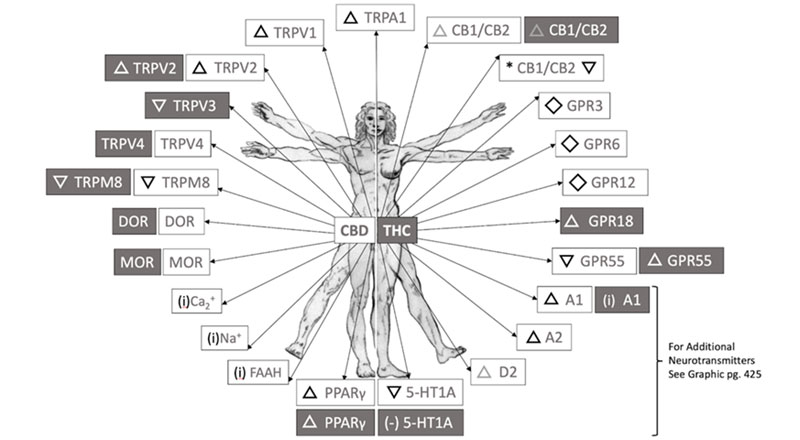
More specifically, this review contains four lab-tests, three animal experiments, eleven reviews, meta-analyses, or expert opinions, and one double-blind placebo-controlled trial, all of which are beginning to offer new understanding and insight.
Laboratory trials:
Brazil (Oct. 2021): The synthetic cannabinoid WIN 55,212-2 reduced the levels of pro-inflammatory cytokines (i.e. IL 6, 8, 18) and tumor necrosis factor-alpha (TNF-α) released by SARS-CoV-2 infected heart cells resulting in reduced cytotoxic damage to the affected tissue.1 South Africa, Nigeria (Sep. 2021): Whole genomic data revealed that cannabinoid-based therapeutics may have the ability to molecularly interact with codon mRNAs of proteins implicated in the replication, translation, assembly, and release of SARS-CoV-2.2
Nigeria (Mar. 2021): Recovery from COVID-19 infection claimed by patients who consumed black seed oil could be linked to the presence of caryophyllene oxide (and/or other compounds contained in the plant).3Taiwan (Jun. 2020): Essential oils of lemon down-regulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells.4
Animal Experiments:
US (Nov. 2021): The endocannabinoid anandamide (AEA) may reduce signs and symptoms of ARDS via direct suppression of inflammation in the lungs and by modulating the microbiota in the lungs and the gut. As such, the authors posit that increasing the bioavailability of AEA through the inhibition of fatty acid amide hydrolase (FAAH), may represent a novel and effective treatment option against ARDS.5
US, Belgium, and Canada (Oct. 2021): CBD was able to reduce hypoxia and pro-inflammatory cytokines in the lungs and blood of the affected test animals thus protecting the lungs. The authors posit that CBC effects via modulation of transient receptor potential (TRP) cation channels (i.e., TRPA1 and TRPV1), increasing their expression by 5-folds in lung tissues re-establishing the homeostasis and immune balance.6
US (Feb. 2022): CBD was able to inhibit infection of SARS-CoV-2 in cells and mice. More specifically, CBD and its metabolite 7-OH-CBD (but not THC) were able to significantly block SARS-CoV-2 replication in lung epithelial cells; CBD acts after viral entry, inhibiting viral gene expression and reversing several effects of SARS-CoV-2 on host gene transcription. Furthermore, CBD (100 mg/ml oral solution) had a significant negative association with positive SARS-CoV-2 tests. As such, CBD may emerge as a potential preventative agent for early-stage SARS-CoV-2 infection.7
Meta-analyses, Reviews, Expert Opinion:
UAE and India (Feb. 2021): Echinacea represents a plausible novel herbal therapeutic of potential value in the treatment context of COVID-19. Its effects on infection, inflammation, and immunity via modulation of CB2 and peroxisome proliferator-activated receptor gamma (PPARγ) receptor sites are highlighted as potential therapeutic target mechanisms. However, the authors also point out that to date no comprehensive and conclusive data is available on the preclinical or clinical benefits of Echinacea in COVID-19.8
US and Jamaica (Feb. 2021): Non-cannabinoid metabolites of cannabis have therapeutic potential such as caflanone (a flavonoid) with selective activity against the human viruses including the coronavirus OC43 (HCov-OC43) responsible for COVID-19 is one of the most promising non-cannabinoid molecules that is being advanced into clinical trials.9
Romania (Mar. 2021): Modulation of several components of the ECS may reduce lung inflammation, reduce fibrosis, decrease viral replication, and may mitigate cytokine storm syndrome, a common complication in this patient population. More specifically, the authors report that activation of the ECS may diminish viral entry and replication, and potentially decrease pro-inflammatory cytokines including IL-2, IL-4, IL-6, IL-12, TNF-α, or IFN-γ.10
Poland (Nov. 2021): This review highlights the potentially relevant therapeutic effects (i.e., anti-inflammatory, antioxidant, and anti-fibrotic properties) of several endo- phyto- and synthetic cannabinoids as well as inhibitors of endocannabinoid degradation in the treatment context of respiratory illness including that of COVID-19 and related complications such as acute respiratory distress syndrome (ARDS), acute lung injury (ALI) and lung ischemia reperfusion injury (LIRI), for example.11
Bangladesh (Dec. 2021): Several cannabinoids may have stable conformations with the binding pocket of the Mpro enzyme of SARS-CoV-2, which has a pivotal role in viral replication and transcription. As such, cannabis-based therapeutics are novel therapeutic candidates for COVID-19.12
Turkey (Aug. 2021): ECS-based modulation, via especially CBD, may be effective in the treatment of patients infected by SARS-CoV-2.13
US (Dec. 2021): CBD significantly inhibits the metabolism of the antiviral drug remdesivir. As such, the authors posit that co-administration of CBD may be a novel approach to enhance its effectiveness in COVID-19 patients.14
China (Nov. 2021): Data revealed that atractyline and shogaol (components of Qing Fei Pai Du decoction commonly used in Traditional Chinese medicine) demonstrated CB2 agonist activities with potential relevance to corona viral diseases.15
China, Nigeria (Oct. 2021): Natural plant-based CBD may be considered to improve prophylaxis and treatment for COVID-19.16
Canada, US (Dec. 2020): Examining potential Covid Drug-to-Cannabinoid interactions (i.e., THC or CBD) this team of researchers found that of the 28 currently available pharmacological treatment candidates for COVID-19 treatment, 12 (i.e., bevacizumab, brilacidin, convalescent plasma, favipiravir, galidesivir [BCX4430], griffithsin, intravenous immunoglobulin, niclosamide, REGN3048, remdesivir, vitamin C, and XueBiJing) had unknown or unlikely drug-to-drug interactions; 10 were found to possibly result in DDIs via CYP interaction; 5 through drug transporter interactions; 6 through protein binding, and 15 that may cause pharmacodynamic-based DDIs. More specifically:
- Anakinra (CBD may increase the risk of infections)
- Arbidol (may increase bioavailability of THC and CBD)
- Azithromycin (CBD may increase risk of diarrhea)
- Baricitinib (CBD may increase risk of infection, malignancy, or thrombosis)
- Chloroquine/hydroxychloroquine (CBD may increase the risk of diarrhea and/or headache)
- Darunavir/cobicistat (CBD may increase risk of headache or diarrhea)
- Disulfiram (THC, CBD may increase risk of sedation)
- Dexamethasone (CBD may increase risk of headache; THC may increase euphoria)
- Eculizumab (CBD may increase the risk of developing a headache)
- Lopinavir/ritonavir (CBD may increase the risk for diarrhea, fatigue, and/or headache; THC may increase dizziness)
- Nelfinavir (CBD may increase risk of headache or diarrhea)
- Nitasoxanide (CBD may increase risk of headache)
- Sarilumab (CBD may increase risk of liver injury)
- Sofosbuvir (CBD may increase the risk for diarrhea, fatigue, and/or headache)
- Tocilizumab (CBD may increase risk of infections if co-administered with immune- or myelosuppressive agents)TZLS-501 (CBD may increase risk of infections if co-administered with immune- or myelosuppressive agents).17
Italy (Sep. 2021): Molecular docking models suggest that pinene, linalool, and limonene target COVID-19 proteins. In addition, after analyzing environmental data the authors posit that ‘forest bathing’ as a therapeutic practice, using nasal sprays containing terpenes, and preserving and increasing forest coverage may have positive impact on this patient population.18
Double-Blind Placebo-controlled Trial:
Israel, Brazil, Canada (Oct. 2021): In this first published clinical trial of this kind physicians co-administered 300 mg of CBD or placebo to standard symptomatic care to ninety-one patients with mild and moderate COVID-19 symptoms for a period of 14 days. Resulting data showed no change in clinical progression of the participating patients.19
Summary
Since our first review (part 1) pre-clinical data has grown significantly indicating potentially new treatment trends involving several components of the endocannabinoidome (ECD) including:
- Endocannabinoids (i.e., anandamide)
- Plant-based cannabinoids (i.e., THC, CBD, CBD’s metabolite 7-OH-CBD, and CBC)
- Synthetic cannabinoids (i.e., WIN55,212-2)
- Endocannabinoid degrading enzymes (i.e., FAAH)
- ECD sensitive receptor sites (i.e., CB2, TRPA1, TRPV1, PPARγ)
- Herbs containing compounds that modulate ECD sensitive receptor sites
- Echinacea
- Black Seed/caryophyllene
- Essential oil of lemon/limonene,
- Caflanone (a flavonoid)
- Linalool
- Eucalyptol
- Pinene
- CBD may produce a treatment synergy with remdesivir
- Aractyline and shogaol both components of Qing Fei Pai Du decoction commonly used in Traditional Chinese medicine may show treatment efficacies via modulation of CB2
- Potential cannabinoid-to-drug interactions: Use caution when co-administering THC or CBD with several pharmaceutical agents commonly used to treat corona viral diseases (see list above and consider consulting the study see annotation)
However, with only one clinical trial completed showing no efficacy of CBD (at the dosages used) any claims of cannabis or cannabinoid-based constituents being a cure in the treatment context of patients with COVID-19 are still unsubstantiated by clinical trials.
Endnotes:
1. Aragão, L., Oliveira, J. T., Temerozo, J. R., Mendes, M. A., Salerno, J. A., Pedrosa, C., Puig-Pijuan, T., Veríssimo, C. P., Ornelas, I. M., Torquato, T., Vitória, G., Sacramento, C. Q., Fintelman-Rodrigues, N., da Silva Gomes Dias, S., Cardoso Soares, V., Souza, L., Karmirian, K., Goto-Silva, L., Biagi, D., Cruvinel, E. M., … Rehen, S. K. (2021). WIN 55,212-2 shows anti-inflammatory and survival properties in human iPSC-derived cardiomyocytes infected with SARS-CoV-2. PeerJ, 9, e12262.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8504461/
2. Erukainure, O. L., Matsabisa, M. G., Muhammad, A., Abarshi, M. M., Amaku, J. F., Katsayal, S. B., & Nde, A. L. (2021). Targeting of Protein’s Messenger RNA for Viral Replication, Assembly and Release in SARS-CoV-2 Using Whole Genomic Data From South Africa: Therapeutic Potentials of Cannabis Sativa L. Frontiers in pharmacology, 12, 736511. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8448283/
3. Duru, C. E., Duru, I. A., & Adegboyega, A. E. (2021). In silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets. Bulletin of the National Research Centre, 45(1), 57.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7952832/
4. Senthil Kumar, K. J., Gokila Vani, M., Wang, C. S., Chen, C. C., Chen, Y. C., Lu, L. P., Huang, C. H., Lai, C. S., & Wang, S. Y. (2020). Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants (Basel, Switzerland), 9(6), 770. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7355681/
5. Sultan, M., Wilson, K., Abdulla, O. A., Busbee, P. B., Hall, A., Carter, T., Singh, N., Chatterjee, S., Nagarkatti, P., & Nagarkatti, M. (2021). Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells, 10(12), 3305.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8699344/
6. Khodadadi, H., Salles, É. L., Shin, E., Jarrahi, A., Costigliola, V., Kumar, P., Yu, J. C., Morgan, J. C., Hess, D. C., Vaibhav, K., Dhandapani, K. M., & Baban, B. (2021). A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. Journal of cannabis research, 3(1), 45.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8485768/
7. Nguyen LC, Yang D, Nicolaescu V, Best TJ, Gula H, Saxena D, Gabbard JD, Chen SN, Ohtsuki T, Friesen JB, Drayman N, Mohamed A, Dann C, Silva D, Robinson-Mailman L, Valdespino A, Stock L, Suárez E, Jones KA, Azizi SA, Demarco JK, Severson WE, Anderson CD, Millis JM, Dickinson BC, Tay S, Oakes SA, Pauli GF, Palmer KE; National COVID Cohort Collaborative Consortium, Meltzer DO, Randall G, Rosner MR. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci Adv. 2022 Feb 25;8(8):eabi6110.
https://www.science.org/doi/10.1126/sciadv.abi6110
8. Nagoor Meeran, M. F., Javed, H., Sharma, C., Goyal, S. N., Kumar, S., Jha, N. K., & Ojha, S. (2021). Can Echinacea be a potential candidate to target immunity, inflammation, and infection – The trinity of coronavirus disease 2019. Heliyon, 7(2), e05990. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7870107/
9. Lowe, H., Steele, B., Bryant, J., Toyang, N., & Ngwa, W. (2021). Non-Cannabinoid Metabolites of Cannabis sativa L. with Therapeutic Potential. Plants (Basel, Switzerland), 10(2), 400. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7923270/
10. Lucaciu, O., Aghiorghiesei, O., Petrescu, N. B., Mirica, I. C., Benea, H., & Apostu, D. (2021). In quest of a new therapeutic approach in COVID-19: the endocannabinoid system. Drug metabolism reviews, 53(4), 478–490.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7989954/
11. Kicman A, Pędzińska-Betiuk A, Kozłowska H. The potential of cannabinoids and inhibitors of endocannabinoid degradation in respiratory diseases. Eur J Pharmacol. 2021 Nov 15;911:174560.
https://pubmed.ncbi.nlm.nih.gov/34648805/
12. Mahmud, M. S., Hossain, M. S., Ahmed, A., Islam, M. Z., Sarker, M. E., & Islam, M. R. (2021). Antimicrobial and Antiviral (SARS-CoV-2) Potential of Cannabinoids and Cannabis sativa: A Comprehensive Review. Molecules (Basel, Switzerland), 26(23), 7216. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8658882/
13. Onay, A., Ertaş, A., Süzerer, V., Yener, İ., Yilmaz, M. A., Ayaz-Tilkat, E., Ekinci, R., Bozhan, N., & Irtegün-Kandemir, S. (2021). Cannabinoids for SARS-CoV-2 and is there evidence of their therapeutic efficacy?. Turkish journal of biology = Turk biyoloji dergisi, 45(4), 570–587. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8573844/
14. Saraswat A, Vartak R, Patki M, Patel K. Cannabidiol Inhibits In Vitro Human Liver Microsomal Metabolism of Remdesivir: A Promising Adjuvant for COVID-19 Treatment. Cannabis Cannabinoid Res. 2021 Dec 16.
https://pubmed.ncbi.nlm.nih.gov/34918945/
15. Xu, F., Hou, T., Shen, A., Jin, H., Xiao, Y., Yu, W., Li, X., Wang, J., Liu, Y., & Liang, X. (2021). Mechanism deconvolution of Qing Fei Pai Du decoction for treatment of Coronavirus Disease 2019 (COVID-19) by label-free integrative pharmacology assays. Journal of ethnopharmacology, 280, 114488.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8329432/
16. Chowdhury, A., Sajid, M., Jahan, N., Adelusi, T. I., Maitra, P., Yin, G., Wu, X., Gao, Y., & Wang, S. (2021). A secondary approach with conventional medicines and supplements to recuperate current COVID-19 status. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 142, 111956.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8313489/
17. Land MH, MacNair L, Thomas BF, Peters EN, Bonn-Miller MO (2020) Letter to the Editor: Possible drug–drug interactions between cannabinoids and candidate COVID-19 drugs, Cannabis and Cannabinoid Research 5:4, 340–343.
https://www.liebertpub.com/doi/10.1089/can.2020.0054
18. Valentina Roviello and Giovanni N. Roviello. Less COVID-19 deaths in southern and insular Italy explained by forest bathing, Mediterranean environment, and antiviral plant volatile organic compounds. Environ Chem Lett. 2021 Sep 1 : 1–11.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8408569/
19. Crippa JAS, Pacheco JC, Zuardi AW, Guimarães FS, Campos AC, Osório FL, Loureiro SR, dos Santos RG, Souza JDS, Ushirohira JM, Ferreira RR, Mancini Costa KC, Scomparin DS, Scarante FF, Pires-Dos-Santos I, Mechoulam R, Kapczinski F, Fonseca BAL, Esposito DLA, Passos ADC, Dal Fabbro AL, Bellissimo-Rodrigues F, Arruda E, Scarpelini S, Andraus MH, Nather Junior JC, Wada DT, Koenigkam-Santos M, Santos AC, Busatto Filho G, Hallak JEC; for the Cannabidiol for COVID-19 Patients (CANDIDATE) Trial Investigators (2021) Cannabidiol for COVID-19 patients with mild to moderate symptoms (CANDIDATE study): a randomized, double-blind, placebo-controlled clinical trial, Cannabis and Cannabinoid Research X:X, 1–12