An Evidence-Based Overview
Terpenes are compounds that are abundantly produced by plants but also occur as a metabolic product in other organisms such as bacteria, fungi, insect, and mammals.1 Not dissimilar to flavonoids, terpenes contribute in a multitude of ways to endow the plant kingdom with support, protection, a means of communication, and of course, its abundant taste, color, and especially, its fragrance. Indeed, terpenes are not just essential for healthy plant life but play critical structural and functional roles in most life forms on Earth.2 For instance, the terpene squalene is one component used to form cholesterol, which in turn plays an essential role in the building of the skin of each of our individual cells, our cellular membranes.3 In contrast, the terpene phytol is able to induce several functional effects associated with its antioxidant, anti-inflammatory, or anti-microbial activity.4 But before we explore the role of terpenes in the context of health, healing, and well-being in detail, it is helpful to briefly review the scientific underpinnings about how we experience scent itself, how the experience can dramatically differ between people, and how it affects our bodies, minds, and emotions.
The Objective Nose
For several decades now biologist thought they had figured out all the intricacies of how scent works. The orthodox model takes our previous “lock and Key” analogy a step further by literally naming the process by which scent molecule fit into corresponding nasal olfactory chemoreceptors in the nose, the “lock and key” mechanism. In this basic model once the connection between a receptor and a fitting key is made, a signal is sent to a specific portion of the brain that recognizes this specific scent and we become aware of it or in other words, we experience its smell. The scent of lemon (from the terpene limonene) is a key that fits into one unique receptor while the terpene pinene fits into another and both correspond to different portions of the brain that differentiate between them. As such, the effects that they induce are different from each other.
However, the process of experiencing scent is perhaps more complex than can be explained by the lock and key mechanism alone. In fact, quantum experiments have added an additional layer of complexity to the lock and key model by showing that beyond that of matching scent or key molecule with a specific scent receptor, the nose also listens to scent molecules in a process that is not unlike the process of hearing (acoustic resonance). In other words, it isn’t just the shape of the keys that are responsible for the experience of a specific scent but also the movement of electron (vibration) jumping between the elements that form each key molecule and as such bind the elements together with novel characteristics. In this context, limonene vibrates at one frequency while pinene, at another. The marvel of this theory is that our experience of smell and by extension the effects to body, mind, and emotion it engenders, has to do with the vibration of molecular bonds and their wavelike behavior and not just the shape of a particular key or scent molecule.
The Subjective Nose
Unlike our other senses, smell has a direct anatomical and functional link with the limbic system responsible for emotion and behavior. As such, scent can directly affect how we feel and behave. And, while the lock and key mechanism will be similar between humans, the resulting associations each scent can trigger can vary greatly. In other words, while many terpene-based scents tend to primarily induce a pleasurable experience for one person, the same scent can trigger discomfort in another. As such, the way we individually experience scent has a cascading secondary influence over our corresponding internal reality. A scent that causes us stress can lead to an increase in stress hormones (e.g. cortisol, various pro-inflammatory cytokines, epinephrine). In contrast, a scent that induces relaxation may work by enhancing serotonin, oxytocin, dopamine, or anandamide levels. Furthermore, the unique ways we experience scent can directly affect our choices and behavior. Will we choose to respond with compassion or act out aggressively? Which may explain, in part, why cannabis is not going to work the same for every single person but is optimized by an individual approach to matching specific cannabis constituents with the needs of each person. In other words, one type of cannabis that resonates well with one person may not vibe well with the next. And, while scent-based effects tend to vary between people here are a few examples where scents have shown to mostly induce measurable therapeutic effects.
The Therapeutic Nose
Scent molecules reach the brain primarily via two distinct pathways: by interacting with either the olfactory or the respiratory systems. Numerous mechanisms have been proposed by which scent molecules reach the brain including:1
- the transmission of signals via the olfactory nerve
- by extra and intra cellular transports
- by crossing the alveolar-capillary barrier
- by crossing the blood brain barrier (BBB)
In the therapeutic setting once scent or scent-induced signals reach the brain, they readily induce specific cellular events we experience as a reduction in stress,2 improvements in mood,3 or as physiological changes associated with potent anti-inflammatory,4 anti-oxidative, 5 and neuroprotective6 effects for example. More specifically, consider the data from 29 clinical trials that have examined terpene-induced beneficial effects across numerous chronic patient populations (see annotated list below).
Terpenes with Proven Effects (Clinical Trials)
| Borneol | Generalized Pain1 Middle Ear Infection2 Stroke/Cerebral Infarction3 |
| Eucalyptol | Anxiety and Panic Disorders4 Asthma5 Bronchitis6 Chronic Obstructive Pulmonary Disease7 Menstrual Pain and Associated Conditions8 |
| Geraniol | Irritable Bowel Syndrome9 |
| Limonene | Brain Cancer10 Breast Cancer11 Cancer- Nonspecific12 Depression13 Pancreatic Cancer14 |
| Linalool | Acute Pain15 Anxiety and Panic Disorders16 Bronchitis17 Depression18 Menstrual Pain and Associated Conditions19 Post-Traumatic Stress Disorder20 Severe Stress Reaction/Oxidative Stress21 Stress and Life Management Difficulty22 |
| Pinene | Chronic Obstructive Pulmonary Disease23 Cough24 Kidney Stones25 Stress and Life Management Difficulty26 |
| ß-Caryophyllene | Bacterial Infections27 Menstrual Pain and Associated Conditions28 Nicotine Dependence and Withdrawal29 |
Terpenes Concentration and Pharmacological Effects
Most terpenes can be detected by the human nose. However, that does not mean that each plays an equally important role in producing a measurable therapeutic effect. Some may not concentrate in large enough amounts to be considered pharmacologically relevant. However, that does not mean they are irrelevant, especially in producing full-spectrum synergies that are perhaps too subtle to be consciously experienced. They too may still play a role in contributing to subtle cascading events with potential effects to our health.
When looking at the currently available scientific evidence about a minimum of terpene concentration it would take to produce a noticeable or measurable effect, we are left with little data to make more discerning decisions. However, in the expert opinion of some researchers and, based on current analytical limits and technological measuring capabilities, a threshold of terpene concentrations of 0.05% or higher is a reasonable mark to expect pharmacological effects in humans.1
Of Practical Relevance: Monoterpenes tend to dominate and may represent up to 10% of fresh trichome content but after cannabis flowers have been dried actual monoterpene yield of most dried flower is significantly less than 1% while sesquiterpenes such as ß-caryophyllene remain stronger in comparison.2
Terpenes — Terpenoids — Volatile Oil — Essential Oil
The usage of terpenes, terpenoids, volatile oil, and essential oil (EO) are often used interchangeably to mean the same or a different thing, which may produce some confusion. The following distinctions clarify the important differences and similarities.
What all four terms have in common is that each:1
- Are concentrated scent molecules
- Readily evaporate in the air
- Do not mix with water
- Readily mix with alcohols, ethers, and other fixed oils (i.e. oils that do not evaporate)
- Are typically colorless and liquid at room temperature
How do they differ:2
- EO’s are typically steam distilled and contain full spectrum organic compounds such as alcohols, aldehydes, amides, amines, esters, ethers, ketones, oxides, phenols, and terpenes.
- Terpenoids and terpenes are typically produced as isolates and as such tend to be close to being pure i.e. containing very little other compounds.
- Terpenes/Terpinoids can be plant derived, derived specifically from cannabis or man-made.
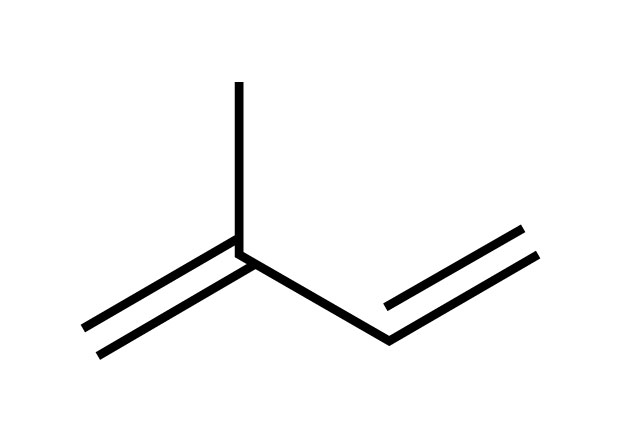
Terpene vs Terpenoid: Conceptually terpenes in their most basic form exist as hemiterpenes formed by a single isoprene unit (see graphic below) whose chemical backbone are 5 carbon atoms. It takes 2 hemiterpenes to make one fully formed terpene called a monoterpene. More complex forms of terpenes are produced by a process called biosynthesis where plants add or combine repeating sets of additional hemiterpenes. For example, the backbone of the monoterpene borneol is formed using two hemiterpenes while the sesquiterpene β-caryophyllene requires three of them. Terpenes are hydrocarbons because they only contain the elements carbon (C) and hydrogen (H) and no oxygen. In contrast, while terpenoids are also formed from single isoprene units, they are not considered hydrocarbons due to a change (aka oxidation) that occurs during the drying process for instance after which in addition to containing the elements C and H they now have gained an additional oxygen element (O).
Essential Oil of Cannabis
EO of cannabis is produced most frequently by steam distillation. It is very rare and one of the most expensive essential oils. EO of cannabis is primarily used in very diluted forms and often mixed with carrier oils for topical applications. It takes large quantities of flowers to produce a drop of essential oil of cannabis. More specifically, it takes 1,000kg of fresh flowers to produce 1.3L of cannabis essential oil, or 1kg to produce 1.3gm.1
Terpenes in Cannabis
Terpenes (like cannabinoids) are present in the small mushrooming, hair-like features or tiny outgrowths (trichomes) of the outer layer of the cannabis flowers and to a lesser degree on the leaves. Trichomes can be seen with the naked eye and upon closer inspection with a magnifying glass can reveal a crystalline structure beneath a coating of sticky, shiny, glass-like appearances.
Depending on specific environmental conditions cannabis will produce different types of terpenes to meet its needs and as such, each terpene is transformed to have unique properties and functions to realize protection against pests, excessive sunlight, or herbivores. Cannabis growers will often adapt the grow environment to maximize the yield of those terpenes they value the most, either to create a specific scent and flavor experience or to work toward specific patient outcomes (see chart of terpenes and conditions that may benefit from their use).
Terpenes Confirmed in Cannabis
Regarding the quantification of cannabis-based terpenes different sources tend to arrive a different total. However, by 2021, 120 cannabis-based terpenes (aka isoprenoids) with the correct chemical structure had been confirmed.1
Terpenes are named according to the number of individual isoprene units used to comprise them. While there are more classes of terpenes in general, here we list only cannabis-based terpene and the class of terpenes to which they belong (alphabetically according to class):
- 61 Monoterpene (10 carbon atoms/2 isoprene units)
- Borneol • bornyl acetate • camphene • camphene hydrate • camphor • Δ3-carene • Δ4-carene • carvacrol • cis-carveol • carvone • 1,4-cineol • 1,8-cineol • citral B • citronellol • p-cymene • p-cymene-8-ol • β-cyclocitral • dehydro-p-cymene • dihydrocarvone • dihydrocarveyl acetate • fenchone • fenchyl alcohol • geranyl acetone • geraniol • ipsdienol • limonene • linalool • linalool oxide • cis-linalool oxide • m-mentha-1,8-(9)-dien-5-ol • methyl-2-heptene-6-one • myrcene • nerol • cis-β-ocimene • trans-β-ocimene • perillene • α-phellandrene • β-phellandrene • 3-phenyl-2-methyl-prop-1-ene • α-pinene • α-pinene oxide • β-pinene • pinocarveol • pinocarvone • piperitenone • piperitenone oxide • piperitone oxide • pulegone • sabinene • sabinene hydrate • cis-sabinene hydrate • sabinol • safranal • thujyl alcohol • α-thujene • α-terpinene • α-terpinolene • γ-terpinene • terpinene-4-ol • α-terpineol • β-terpineol
- 51 Sesquiterpene (15 carbon atoms/3 isoprene units)
- Allo-aromadendrene • α-cis-bergamotene • α-trans-bergamotene • β-bisabolene • γ-cis-bisabolene • γ-trans-bisabolene • α-bisabolol • epi-α-bisabolol • α-cadinene • δ-cadinene • γ-cadinene • calamenene • α-caryophyllene (α-humulene) • β-caryophyllene • caryophyllene alcohol (caryophyllenol) • caryophyllene oxide • iso-caryophyllene • α-cedrene • clovandiol • α-copaene • α-cubebene • curcumene • γ-curcumene • β-elemene • γ-elemene • α-eudesmol • β-eudesmol • γ-eudesmol • β-farnesene • trans-trans-α-farnesene • (Z)-β-farnesene • farnesol • farnesyl acetone • germacrene-B • α-guaiene • guaiol • α-gurjunene • humulene epoxide I • humulene epoxide II • ledol • longifolene • α-longipinene • γ-muurolene • nerolidol • epi-β-santalene • selina-3,7(11)-diene • selina-4(14),7(11)-diene • α-selinene • β-selinene • viridiflorene • α-ylangene
- 2 Diterpenes (20 carbon atoms/4 isoprene units)
- Phytol • neophytadiene
- 2 Triterpene (30 carbon atoms/6 isoprene units)
- Friedelin • epifriedelanol
- 4 Miscellaneous Cannabis Terpenes
- Vomifoliol • dihydrovomifoliol • β-ionone • dihydroactinidiolide
Endnotes Terpenes
A World of Scent
1. Jansen B, de Groot. A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat Product Rep. (2004) 21:449–77.
2. Oldfield, E., & Lin, F. Y. (2012). Terpene biosynthesis: modularity rules. Angewandte Chemie (International ed. in English), 51(5), 1124–1137.
3. Ibid.
4. Islam MT, Ali ES, Uddin SJ, Shaw S, Islam MA, Ahmed MI, Chandra Shill M, Karmakar UK, Yarla NS, Khan IN, Billah MM, Pieczynska MD, Zengin G, Malainer C, Nicoletti F, Gulei D, Berindan-Neagoe I, Apostolov A, Banach M, Yeung AWK, El-Demerdash A, Xiao J, Dey P, Yele S, Jóźwik A, Strzałkowska N, Marchewka J, Rengasamy KRR, Horbańczuk J, Kamal MA, Mubarak MS, Mishra SK, Shilpi JA, Atanasov AG. Phytol: A review of biomedical activities. Food Chem Toxicol. 2018 Nov;121:82-94.
The Therapeutic Nose
1. Satou T, Hayakawa M, Kasuya H, Masuo Y, Koike K. (2017) Mouse brain concentrations of α-pinene, limonene, linalool, and 1,8-cineole following inhalation. Flavour Fragr J. 32:36–9.
Fung, T., Lau, B., Ngai, S., & Tsang, H. (2021). Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. International journal of molecular sciences, 22(9), 4844.
Selvaraj K, Gowthamarajan K, Karri VVSR. (2018 Dec) Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol. 46(8):2088-2095.
Agatonovic-Kustrin, S., Kustrin, E., & Morton, D. W. (2019). Essential oils and functional herbs for healthy aging. Neural regeneration research, 14(3), 441–445.
2. Ebrahimi H, Mardani A, Basirinezhad MH, Hamidzadeh A, Eskandari F. (2021 Jan 9) The effects of Lavender and Chamomile essential oil inhalation aromatherapy on depression, anxiety and stress in older community-dwelling people: A randomized controlled trial. Explore (NY). S1550-8307(21)00001-X.
Karan NB. (2019 Nov 1) Influence of lavender oil inhalation on vital signs and anxiety: A randomized clinical trial. Physiol Behav. 211:112676.
3. Ebrahimi H, Mardani A, Basirinezhad MH, Hamidzadeh A, Eskandari F. (2021 Jan 9) The effects of Lavender and Chamomile essential oil inhalation aromatherapy on depression, anxiety and stress in older community-dwelling people: A randomized controlled trial. Explore (NY). S1550-8307(21)00001-X.
Karan NB. (2019 Nov 1) Influence of lavender oil inhalation on vital signs and anxiety: A randomized clinical trial. Physiol Behav. 211:112676.
Şentürk A, Tekinsoy Kartın P. (2018 Nov/Dec) The Effect of Lavender Oil Application via Inhalation Pathway on Hemodialysis Patients’ Anxiety Level and Sleep Quality. Holist Nurs Pract. 32(6):324-335.
4. Kim DS, Lee HJ, Jeon YD, Kee JY, Kim HJ, Shin XJ, et al. (2015) Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chinese Med. 43:731–42.
Khoshnazar M, Bigdeli MR, Parvardeh S, Pouriran R. (2019 Nov) Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J Pharm Pharmacol. 71(11):1725-1733.
5. Atsumi T, Tonosaki K. (2007 Feb 28) Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 150(1):89-96.
6. Oboh G, Olasehinde TA, Ademosun AO. (2014) Essential oil from lemon peels inhibit key enzymes linked to neurodegenerative conditions and pro-oxidant induced lipid peroxidation. J Oleo Sci63:373–81.
Terpenes with Proven Effects (Clinical Trials)
1. Wang, S., Zhang, D., Hu, J., Jia, Q., Xu, W., Su, D., Song, H., Xu, Z., Cui, J., Zhou, M., Yang, J., & Xiao, J. (2017). A clinical and mechanistic study of topical borneol-induced analgesia. EMBO molecular medicine, 9(6), 802–815. 2
2. Liu SL. Therapeutic effects of borneol-walnut oil in the treatment of purulent otitis media. (1990 Feb) Zhong Xi Yi Jie He Za Zhi. 10(2):93-5, 69.
3. Chen, Z. X., Xu, Q. Q., Shan, C. S., Shi, Y. H., Wang, Y., Chang, R. C., & Zheng, G. Q. (2019). Borneol for Regulating the Permeability of the Blood-Brain Barrier in Experimental Ischemic Stroke: Preclinical Evidence and Possible Mechanism. Oxidative medicine and cellular longevity, 2019, 2936737.
4. Kasper S, Gastpar M, Müller WE, Volz HP, Möller HJ, Schläfke S, Dienel A. Lavender oil preparation Silexan is effective in generalized anxiety disorder–a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol. 2014 Jun;17(6):859-69.
5. Juergens UR, Stöber M, Vetter H. (1998 Nov 17) Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur J Med Res. 3(11):508-10.
Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. (2003 Mar) Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 97(3):250-6.
Worth H, Dethlefsen U. (2012 Oct) Patients with asthma benefit from concomitant therapy with cineole: a placebo-controlled, double-blind trial. J Asthma. 49(8):849-53.
Juergens UR. (2014 Dec) Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res (Stuttg). 64(12):638-46.
Yadav N, Chandra H (2017) Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS ONE 12(11): e0188232.
6. Fischer, J., & Dethlefsen, U. (2013). Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough (London, England), 9(1), 25.
Kähler, C., Derezinski, T., Bocian-Sobkowska, J., Keckeis, A., & Zacke, G. (2019). Spicae aetheroleum in uncomplicated acute bronchitis: a double-blind, randomised clinical trial. Wiener medizinische Wochenschrift (1946), 169(5-6), 137–148.
7. Worth H, Schacher C, Dethlefsen U. (2009 Jul) Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: a placebo-controlled double-blind trial. Respiratory Research. 10:69.
Dorow P, Weiss T, Felix R, Schmutzler H. (1987 Dec) Einfluss eines Sekretolytikums und einer Kombination von Pinen, Limonen und Cineol auf die mukoziliare Clearance bei Patienten mit chronisch obstruktiver Atemwegserkrankung [Effect of a secretolytic and a combination of pinene, limonene and cineole on mucociliary clearance in patients with chronic obstructive pulmonary disease]. Arzneimittelforschung. 37(12):1378-81.
8. Ou MC, Hsu TF, Lai AC, Lin YT, Lin CC. (2012 May) Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial. J Obstet Gynaecol Res. 38(5):817-22.
9. Rizzello, F., Ricci, C., Scandella, M., Cavazza, E., Giovanardi, E., Valerii, M. C., Campieri, M., Comparone, A., De Fazio, L., Candela, M., Turroni, S., & Spisni, E. (2018). Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: a pilot study. BMC complementary and alternative medicine, 18(1), 338.
10. da Fonseca CO, Schwartsmann G, Fischer J, Nagel J, Futuro D, Quirico-Santos T, Gattass CR. (2008 Sep) Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol. 70(3):259-66; discussion 266-7.
da Fonseca CO, Simão M, Lins IR, Caetano RO, Futuro D, Quirico-Santos T. (2011 Feb) Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol. 137(2):287-93.
11. Vigushin DM, Poon GK, Boddy A, English J, Halbert GW, Pagonis C, Jarman M, Coombes RC. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol. 1998;42(2):111-7.
Miller, J. A., Lang, J. E., Ley, M., Nagle, R., Hsu, C. H., Thompson, P. A., Cordova, C., Waer, A., & Chow, H. H. (2013). Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer prevention research (Philadelphia, Pa.), 6(6), 577–584.
12. Azzoli CG, Miller VA, Ng KK, Krug LM, Spriggs DR, Tong WP, Riedel ER, Kris MG. (2003 Jun) A phase I trial of perillyl alcohol in patients with advanced solid tumors. Cancer Chemother Pharmacol. 51(6):493-8.
Bailey HH, Wilding G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Marnocha R, Holstein SA, Stewart J, Lewis KA, Hohl RJ. (2004 Oct) A phase I trial of perillyl alcohol administered four times daily for 14 days out of 28 days. Cancer Chemother Pharmacol. 54(4):368-76.
13. Komori T, Fujiwara R, Tanida M, Nomura J, Yokoyama MM. (1995 May-Jun) Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation. 2(3):174-80.
14. Matos JM, Schmidt CM, Thomas HJ, Cummings OW, Wiebke EA, Madura JA, Patrick LJ Sr, Crowell PL. (2008 Jun) A pilot study of perillyl alcohol in pancreatic cancer. J Surg Res. 147(2):194-9.
15. Kim JT, Ren CJ, Fielding GA, Pitti A, Kasumi T, Wajda M, Lebovits A, Bekker A. (2007 Jul) Treatment with lavender aromatherapy in the post-anesthesia care unit reduces opioid requirements of morbidly obese patients undergoing laparoscopic adjustable gastric banding. Obes Surg. 17(7):920-5.
16. Kasper S, Gastpar M, Müller WE, Volz HP, Möller HJ, Dienel A, Schläfke S. (2010 Sep) Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: a randomized, double-blind, placebo controlled trial. Int Clin Psychopharmacol. 25(5):277-87.
Pia Baldinger, MD, Anna S. Höflich, MD, Markus Mitterhauser, MSc, PhD, Andreas Hahn, MSc, PhD, Christina Rami-Mark, MSc, Marie Spies, MD, Wolfgang Wadsak, MSc, PhD, Rupert Lanzenberger, MD, Siegfried Kasper, MD, (February 2015) Effects of Silexan on the Serotonin-1A Receptor and Microstructure of the Human Brain: A Randomized, Placebo-Controlled, Double-Blind, Cross-Over Study with Molecular and Structural Neuroimaging, International Journal of Neuropsychopharmacology, Volume 18, Issue 4, pyu063.
Kasper S, Möller HJ, Volz HP, Schläfke S, Dienel A. (2017 Jul) Silexan in generalized anxiety disorder: investigation of the therapeutic dosage range in a pooled data set. Int Clin Psychopharmacol. 32(4):195-204.
17. Kähler, C., Derezinski, T., Bocian-Sobkowska, J., Keckeis, A., & Zacke, G. (2019). Spicae aetheroleum in uncomplicated acute bronchitis: a double-blind, randomised clinical trial. Wiener medizinische Wochenschrift (1946), 169(5-6), 137–148.
18. Kasper S, Volz HP, Dienel A, Schläfke S. (2016 Feb) Efficacy of Silexan in mixed anxiety-depression–A randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 26(2):331-340.
19. Ou MC, Hsu TF, Lai AC, Lin YT, Lin CC. (2012 May) Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial. J Obstet Gynaecol Res. 38(5):817-22.
20. Uehleke B, Schaper S, Dienel A, Schlaefke S, Stange R. (2012 Jun 15) Phase II trial on the effects of Silexan in patients with neurasthenia, post-traumatic stress disorder or somatization disorder. Phytomedicine. 19(8-9):665-71.
21. Seol, G. H., Kang, P., Lee, H. S., & Seol, G. H. (2016). Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC neurology, 16, 17.
22. Heuberger E, Redhammer S, Buchbauer G. (2004 Oct) Transdermal absorption of (-)-linalool induces autonomic deactivation but has no impact on ratings of well-being in humans. Neuropsychopharmacology. 29(10):1925-32.
Huang L, Capdevila L. (2017 Mar) Aromatherapy Improves Work Performance Through Balancing the Autonomic Nervous System. J Altern Complement Med. 23(3):214-221.
23. Dorow P, Weiss T, Felix R, Schmutzler H. (1987 Dec) Einfluss eines Sekretolytikums und einer Kombination von Pinen, Limonen und Cineol auf die mukoziliare Clearance bei Patienten mit chronisch obstruktiver Atemwegserkrankung [Effect of a secretolytic and a combination of pinene, limonene and cineole on mucociliary clearance in patients with chronic obstructive pulmonary disease]. Arzneimittelforschung. 37(12):1378-81.
24. Falk AA, Hagberg MT, Löf AE, Wigaeus-Hjelm EM, Wang ZP. (1990 Oct) Uptake, distribution and elimination of alpha-pinene in man after exposure by inhalation. Scand J Work Environ Health. 16(5):372-8.
25. Kim, D. H., Goh, H. J., Lee, H. W., Kim, K. S., Kim, Y. T., Moon, H. S., Lee, S. W., & Park, S. Y. (2014). The effect of terpene combination on ureter calculus expulsion after extracorporeal shock wave lithotripsy. Korean journal of urology, 55(1), 36–40.
Engelstein D, Kahan E, Servadio C. Rowatinex for the treatment of ureterolithiasis. J Urol (Paris). 1992;98(2):98-100.
26. Li Q, Kobayashi M, Wakayama Y, Inagaki H, Katsumata M, Hirata Y, Hirata K, Shimizu T, Kawada T, Park BJ, Ohira T, Kagawa T, Miyazaki Y. (2009 Oct-Dec) Effect of phytoncide from trees on human natural killer cell function. Int J Immunopathol Pharmacol. 22(4):951-9.
Gulluni, N., Re, T., Loiacono, I., Lanzo, G., Gori, L., Macchi, C., Epifani, F., Bragazzi, N., & Firenzuoli, F. (2018). Cannabis Essential Oil: A Preliminary Study for the Evaluation of the Brain Effects. Evidence-based complementary and alternative medicine : eCAM, 1709182.
27. Shim HI, Song DJ, Shin CM, Yoon H, Park YS, Kim N, Lee DH. (2019 Oct 25; ) [Inhibitory Effects of β-caryophyllene on Helicobacter pylori Infection: A Randomized Double-blind, Placebo-controlled Study]. Korean J Gastroenterol. 74(4):199-204.
28. Ou MC, Hsu TF, Lai AC, Lin YT, Lin CC. (2012 May) Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial. J Obstet Gynaecol Res. 38(5):817-22.
29. Rose JE, Behm FM. (1994 Feb) Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 34(3):225-9.
Terpenes Concentration and Pharmacological Effects
1. Adams TB, Taylor SV. Safety evaluation of essential oils: a constituent-based approach. In: Baser KHC, Buchbauer G, editors. Handbook of Essential Oils: Science, Technology, and Applications. Boca Raton, FL: CRC Press; 2010. pp. 185–208, specifically page 192.
2. Ross SA, ElSohly MA. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J Nat Prod. 1996;59:49–51.
Terpenes — Terpenoids — Volatile Oil — Essential Oil
1. Dhifi, W., Bellili, S., Jazi, S., Bahloul, N., & Mnif, W. (2016). Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines (Basel, Switzerland), 3(4), 25.
2. Ibid.
Essential Oil of Cannabis
1. Vito Mediavilla and Simon Steinemann. Essential oil of Cannabis sativa L. strains. Swiss Federal Research Station for Agroecology and Agriculture, Reckenholzstrasse 191, 8046 Zurich, Switzerland.
Terpenes Confirmed in Cannabis
1. Radwan, M. M., Chandra, S., Gul, S., & ElSohly, M. A. (2021). Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules (Basel, Switzerland), 26(9), 2774.


