Similarly to synthetic pharmaceutical versions of cannabis, such as Marinol or nabilone, there are several other artificial groupings of cannabinoids worth noting. You may ask why research using synthetics is necessary. The two most important reasons why it is helpful to explore synthetic cannabinoid-based research are safety and novel research trends.
Synthetic Cannabinoids and Their Clinical Relevance
Safety
The First is safety. The irresponsible use of synthetic cannabinoids can have deadly consequences. As such, responsible healthcare providers or educators will want to understand the forms and potential impact of products containing them. For instance, synthetics have shown up in “street drugs” such as “Spice,” K2, or synthetic marijuana.
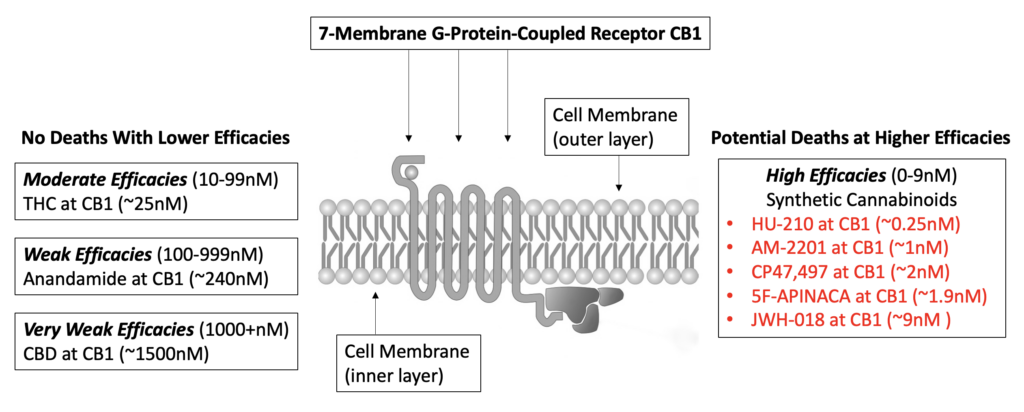
You may have seen news reports that implicate synthetic cannabinoids in overdoses so severe that they cause organ failure and death. The reason why many synthetic cannabinoids are dangerous when compared to their naturally occurring counterparts has to do with the concept of binding efficacy. Naturally occurring and synthetic cannabinoids bind with endocannabinoid receptor sites such as CB1. However, the latter binds with significantly higher efficacy, with direct and potentially devastating consequences. As you can see in the graphic (below), one of the naturally occurring cannabinoids with the highest affinity at CB1 is THC. When comparing THC with HU-210, you will notice that THC binds with moderate affinity, which is high enough (no pun intended) to induce changes in cognition (even hallucinations) but not high enough to kill. In direct contrast, the efficacy of HU-210 (a purported ingredient in “Spice”) is about one hundred times more potent at the same receptor site. Thus it can cause increasingly powerful effects on the central nervous system and any other bodily system that contains CB1 receptors.
Novel Research Trends
Secondly, research insights gained from the responsible use of synthetics in various research models continue to highlight novel benefits of engaging the ECS to create specific effects that have relevance in the clinical setting that has not previously been understood. For instance, CB2 agonism induced via JWH-133 (a full agonist at CB2) has provided more specific insights as to how natural anti-inflammatory cannabinoids such as THC induce related effects that are valued by a significant number of primarily chronic patient populations that share an inflammatory pathology.
CannaKeys has created access to the following ten synthetic cannabinoids with sufficient scientific data to have potential relevance in the clinical setting.
CannaKeys Synthetic Cannabinoids
- JWH-x Synthetic Cannabinoids (2)
- SR-x Synthetic Cannabinoids (2)
- 5F-x Synthetic Cannabinoids
- AB-x Synthetic Cannabinoids
- Abnormal Cannabidiol (Abn-CBD)
- AM-x Synthetic Cannabinoids
- CP-x Synthetic Cannabinoids
- HU-x Synthetic Cannabinoids
- O-x Synthetic Cannabinoids
- WIN-x Synthetic Cannabinoids
Below you’ll find a brief paragraph describing each of the synthetic groups we currently track at CK.
The JWH group of compounds is named after the organic chemist John William Huffman (1932–2022). Huffman and his team have developed over 400 synthetic cannabinoid compounds, most of which are used to study the endocannabinoid system (ECS) and its potential for medicinal application. However, members of the JWH series contain several compounds that have been used to produce “synthetic cannabinoids,” such as “Spice” or “K2,” which have been implemented in several well-publicized overdoses and fatalities. As such, many of these compounds are banned in several countries, such as China, Australia, and Ireland.
The SR-x group of synthetic cannabinoids includes rimonabant, first described and marketed by Sanofi as a drug to combat obesity. Also known as SR-141716A, the compound is an orally active selective antagonist at CB1 receptor sites. Rimonabant was sold as an effective weight loss drug in Europe until adverse effects (e.g., depression and suicidal tendencies) forced their makers to pull the drug from the market. It is extensively used to research the endocannabinoid system’s function (ECS).
The 5F-class, including 5F-MDMB-PICA, emerged as a novel “herbal” ingredient in drugs of the European recreational market in 2016 (under the names of, e.g., “K2” and “Spice”) and was the most commonly available synthetic cannabinoid in the USA between 2019 and 2020. However, it is among the more potent members and is associated with severe adverse health, behavioral effects, and deaths.
The AB-x class of synthetic cannabinoids is small. However, two of its prominent members are AB-FUBINACA and AB-CHFUPYCA, both of which were first detected in illegal recreational products in Japan.
Abnormal CBD (Abn-CBD) is a synthetic isomer of CBD and was discovered in 1974. However, a deeper scientific understanding of abnormal CBD, especially in treating human illness, remains mainly wanting.
The AM-x class of synthetic cannabinoids was primarily developed, characterized, and subsequently named after Alexandros Makriyannis, a professor of biochemistry at Northeastern University in Boston.
The AM family of cannabinoids consists of over 40 individual members that produce diverse and varying effects via modulating various components of the ECS. Some members are also analogs with compounds classified under different headings, such as AM-678, aka JWH-018.
The CP-class of synthetic cannabinoids is named after the company Carl Pfizer where they were first synthesized. CP-55,940 was discovered in 1974 and is one of the most studied members of this class. It binds to the same classical endocannabinoid receptor sites, i.e., CB1 and CB2, as THC, albeit with stronger efficacies.
The HU-x series are synthetic cannabinoids that carry the initials based on their place of discovery Hebrew University, Israel.
Initially developed and characterized by Mechoulam et al. (1988), HU-210 and HU-211 are two of several diverse members of the HU family that have attracted significant attention in the international research community. For instance, HU-210 has full agonist activity at CB1 and CB2, while the (+) enantiomer, i.e., HU-211, does not, but instead act via different pathways such as an N-Methyl-D-aspartate (NMDA) antagonist.
The O-x class of synthetic cannabinoids contains diverse members, many of whose effects and binding potential are still subject to ongoing scientific exploration.
WIN 55,212-2 is the most studied compound in the WIN-x group of synthetic cannabinoids. Other members on the CK primary study list include WIN 55-940 (full agonist at CB1 and CB2).

