The reason why cannabis can work so very well for so many different patient populations is that it affects the body and the mind in such a way as to support and fortify the body’s capacity for self-healing and resilience. The right type of cannabis matched to meet each patient’s unique needs and, at the appropriate dose, provides three general holistic mind-body benefits. First, it induces deep relaxation, termed relaxation response (H. Benson et al., 1975).1 Second, it makes it easier to realize positive affect (aka an uplift in mood or a trend toward happiness). Moreover third, in the right setting and with supportive intentions, it is effortless to realize a state of flow of mental-emotional experiences of all kinds—stressful and pleasant. This combination contributes to a complex web of therapeutic effects that benefit over 250 different patient populations,2 many of whom experience chronic health issues.
Before we explore emerging treatment options, the gentle reader may find it instructive to review the origin and endocannabinoid terms, which will help better understand the following material. For people who already understand these concepts, skip the following three paragraphs.
In the late eighties, three international research teams discovered and reported various aspects of the endocannabinoid system (ECS) (W. Devane et al., 1988; L. Matsuda et al., 1990; S. Munro et al., 1993), which led to the rise of a new frontier in medicine, the cannabinoid health science.3
The prefix endo- is taken from the Greek éndon, meaning inner or within; the middle section references cannabis, and the suffix -oid is based on the Greek –oeidḗs meaning like. As such, the term endocannabinoid describes compounds that are like cannabis but are made by the human body.
In the most basic terms, the components of the classical endocannabinoid system (ECS) include:
• Endocannabinoid receptor sites (i.e., GPRs CB1 and CB2)
• Endocannabinoids (i.e., Anandamide and 2-AG)
• Metabolizing enzymes (i.e., FAAH and MAGL)
• Endocannabinoid tone
Let us consider that an adult human body consists of roughly 30 trillion individual cells that organize into eleven organ systems. Many diverse receptor sites are embedded in individual cellular membranes; some of those most abundant include endocannabinoid receptor sites (A. Irving et al., 2008).4 Receptors communicate and respond to meet the need of a constantly changing internal and external environment. As such, each cell plays a never-ending part in transforming a constant cacophony of stressors, change, and chaos into a grand symphony to assure homeostasis, survival, resilience, and by extension, our well-being. And the ECS fills the role of a conductor.
Let us take a specific example. The scientific literature has demonstrated that specific cannabinoid-based therapeutics can work well for patients with post-traumatic stress disorder (PTSD). As previously mentioned, but worth repeating, a therapeutic cannabis experience can initiate a deep sense of relaxation, a gentle uplift in mood, and a state of flow that allows patients to be present with emotional material that may otherwise be intolerable. It enables us to attend to our experience without engaging in coping mechanisms such as negative self-talk, recoiling with dread, or acting out aggressively.
Similarly, ancient Eastern traditions have long used various meditative techniques to induce relaxation response (calmness of mind), positive affect (serenity of mind), and a state of flow (learning to let go of attachments, reducing reactivity). In considering these shared goals, to bring balance or harmony to the holistic self and, with it, significant and positive changes, lasting healing, insights, and connecting with something larger than oneself, the importance of the crosstalk between the endocannabinoid system (ECS) and mind-body medicine (MBM) becomes more defined. Regular access to relaxation, positive affect, and state of flow are markers of a healthy and resilient ECS, promoting overall wellness—a circular self-supporting healing mechanism.
In direct contrast, an ECS overwhelmed by psychophysiological stressors such as unresolved mental-emotional materials (e.g., repressed emotions, cognitive dissonance, self-destructive behaviors), a tendency toward negative affect (e.g., chronic fear, anxiety, aggression), a lack of flow (e.g., years of emotional baggage, limiting or unhealthy beliefs, narrow-mindedness, negative self-talk), an unhealthy diet (e.g., lack of omega-3s), the lack of exercise, or the ill effects of pathological aging (in opposition to optimal aging), all diminishes function and therapeutic capacity—a circular self-destructive mechanism.
In such a situation, the psychophysiological crosstalk between ECS-engaging therapeutics and mind-body approaches can help to re-establish balance and return the patient to a state of health by providing the ECS and, by extension, the whole organism with what it is missing.
Another practical example. One of the fastest-growing patient populations in the cannabis clinic is seniors. A large segment of this population experiences chronic pain, which tends to respond well to cannabinoid-based therapy. Unfortunately, treatment and outcomes still suffer from outdated mindsets, beliefs, and misinformation about cannabis, which are prevalent in this group. While seniors hope that cannabis can mitigate their pain, many still view cannabis as a “dangerous gateway drug” that gets people “high,” produces addiction, and has otherwise dangerous side effects–beliefs that induce internal conflict (cognitive dissonance). Like any stress, anxiety about cannabis can lower pain thresholds, rendering cannabinoid therapy clinically counterproductive (G. Zheng et al., 2015; S. Linton, 2000; A. Hall et al., 2011; A. Johnson et al., 2014).5 However, shifting the mind-body environment through fact-based education before treatment is relatively easy and has the potential to lead to positive clinical outcomes (K. Reiner et al., 2016).6 In other words, relieving anxiety around cannabis through education mitigates its adverse impacts on pain thresholds.
One practical and evidence-based way to reduce patient stress and anxiety is to let seniors know that different kinds of cannabis will induce therapeutic effects as different as night and day. Three types of cannabis and cannabis-based prescription drugs are defined by their ratio of THC to CBD, the two most abundant cannabis constituents. Also called cannabis chemotypes, the Roman numerals I, II, and III are used to distinguish them. A chemotype I contains more THC than CBD, a chemotype II contains relatively equal amounts, and a chemotype III contains more CBD than THC. Each type produces different therapeutic effects valued by specific patient populations that can benefit from their unique ratios. For example, cannabis chemotype III (abundant in CBD with less than 0.3% THC) induces numerous therapeutic effects commonly prescribed to treat multiple mood disorders and pediatric seizures without being addictive, causing changes in cognition, or leading to THC-based adverse effects.
Beyond understanding why cannabis-type-based therapeutics can work so well for specific but diverse groups of chronic patient populations, it may also be helpful to take a more focused perspective on the overlapping and mutually supportive relationship between the ECS and mind-body medicine (MBM).
Mind-Body Approaches and the Endocannabinoidome
Mind-Body Medicine: What is mind-body medicine (MBM)? Depending on who is asked, answers to the question can range from the general (treatment that affects the body and the mind) to the technical (a stress-modulating mechanism that involves the hypothalamus-pituitary-adrenal axis), to the esoteric (an opportunity or a demand for change based on the symptomatic expression of the body’s innate intelligence).
However, the impact of cannabinoid-based products is much more complex than any definition of MBM. It is also more complicated than merely modulating components of the classical ECS when inducing relaxation and being well.
The list below contains naturally occurring molecules that affect the body, mind, and emotions. What is interesting to note is that each of them is, either in part or significantly modulated by the activation of various components of the ECS. The emerging treatment possibilities cannot be underestimated since many psychiatric as well as neurological disorders have identified these varied molecules and activation pathways as targets for pharmaceutical treatment (see incomplete list below):
- Acetylcholine (e.g., memory, wakefulness)7 E. Murillo-Rodríguez et al., 2018
- Cortisol (e.g., stress response)8 Kathmann et al., 2006
- Cytokines (anti-inflammatory)9 L. Jean-Gilles et al., 2010
- Cytokines (pro-inflammatory)10 F. Zádor et al., 2021
- Dopamine (e.g., motivation, reward)11 A. Terzian et al., 2011
- Endogenous opioids (e.g., analgesia)12 M. Kathmann et al., 2006
- Epinephrine (e.g., fight, flight, or freeze)13 N. Niederhoffer et al., 2001
- Gamma-aminobutyric acid (GABA) (e.g., calming)14 S. Lee et al., 2010
- Ghrelin (e.g., hunger, anger)15 L. Senin et al., 2013
- Glucagon (e.g., hungry, angry)16 K. Patel et al., 2014
- Glutamate (e.g., excitement, excitotoxicity)17 A. Köfalvi et al., 2020
- Insulin (e.g., hungry, angry)18 A. Laguerre et al., 2021
- Leptin (e.g., satiated)19 B. Bosier et al., 2013
- Norepinephrine (e.g., stress, attention)20 R. Wyrofsky et al., 2019
- Oxytocin (e.g., trust, intimacy)21 D. Wei et al., 2015
- Serotonin (e.g., happiness, well-being)22 I. Ibarra-Lecue et al., 2021
- Testosterone (e.g., confidence, aggression)23 J. Lim et al., 2023
- Vasopressin (e.g., social recognition, aggression)24 V. Luce et al., 2014
Indeed, targeting specific aspects of the ECS and the overlapping physiological and mental-emotional pathways listed above can significantly mitigate the underlying pathology of many chronic conditions and support the physiology of wellness. It may even allow for a reduction in the number and dosages of prescription drugs used, thus resulting in a reduced adverse effects potential that rises with every additional medication prescribed and potentially significant monetary savings.
This more significant, more complex environment that responds to targeted treatments of the ECS has been termed the endocannabinoidome (eCBome). The relatively new idea of an (eCBome) describes the larger environment interacting with all knowns aspects of the endocannabinoid system (ECS).
We can activate the eCBome via the use of known mediators, including the use of cannabis constituents, but also food (e.g., diet), nutraceuticals (e.g., turmeric), mind-body approaches (e.g., mindfulness, exercise, acupuncture), feeding of the microbiome (e.g., probiotics), and modulating the lipidome (e.g., omega-3, C15).
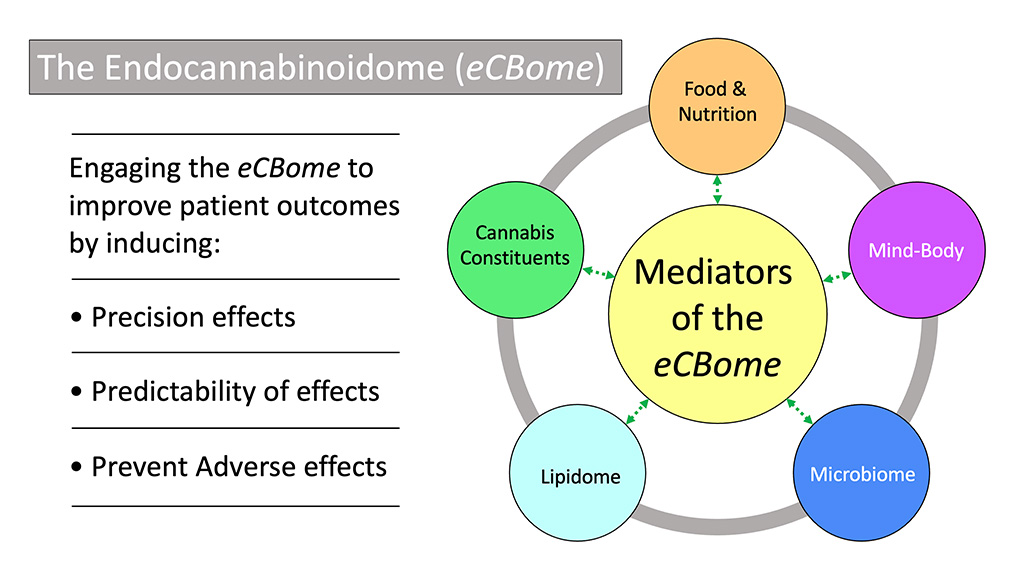
By engaging the eCBome, especially alongside the use of appropriate cannabis-based products, we can significantly and safely improve patient outcomes by:
- Inducing precision effects
- Establishing predictability of effects
- Preventing adverse effects
- Reducing total mg amounts of cannabis-based products used to achieve the same or better and longer-lasting results
Novel Treatment Approaches: Mediators of the eCBome
Mind-Body Medicine (MBM): Meditation, fasting, prayer, or sacramental ritual alongside cannabis use has been used across cultures for millennia to experience bliss and mystical insights to mitigate “dichotomies, polarities, and conflicts of life” (M. Ferrara 2021).25 Recent scientific insights have provided a better understanding of why these practices have such staying power, which brings us to the first eCBome mediator, i.e., MBM.
Meditation improves endocannabinoid tone (V. Brugnatelli et al., 2021).26 Practicing yoga increases levels of endocannabinoids with improvements in mental health (S. Sadhasivam et al., 2020).27 Developing emotional intelligence may optimize ECS-based signaling (R. de Melo Reis et al., 2021).28 And, setting an intention when utilizing cannabis is a significant predictor for the experiences of insight, connectedness, joy, love, and unity with transcendent forces (P. Johnstad, 2020).29
Microbiome: The “human superorganism” concept is often used to describe the microbiome. The human superorganism consists of the human body (the host) and the wide variety of microbial organisms (e.g., viruses, bacteria, fungi) living within and on the skin’s surface. The microbiome’s composition in the gut holds significance for the host’s health and resilience.
Some microbial members of the “superorganism” are disruptive intruders, such as dermatophytes or fungi that cause onychomycosis (foot and nail fungus). In contrast, other beneficial organisms include probiotics (e.g., Acidophilus, Bifidum, Akkermansia muciniphila), which support the host’s health and well-being. Most fermented foods such as sauerkraut, kimchi, kefir, and yogurt naturally contain an abundance of probiotic cultures.
Hippocrates says, “All disease begins in the gut.” We now know that the gut contains an abundance of all components of the classical ECS and a diverse group of neurotransmitters, hormones, and other communication molecules that are part of the eCBome, such as serotonin, which plays significant roles in gut-brain signaling. Healthy ECS signaling is associated with normal gastrointestinal function, while alterations make us vulnerable to dysfunction and the development of gastrointestinal pathologies.
More specifically, research shows that cross-talk between the ECS and the microbiome improves stress resilience (R. Srivastava et al., 2022),30 which may benefit patients with mood disorders, including PTSD. In addition, the use of probiotics helps to improve endocannabinoid tone (V. Brugnatelli et al., 2021),31 and the oral administration of Lactobacillus acidophilus (common in live yogurt cultures) led to an upregulation of both μ-opioid and CB2 expression with potential implications for gastrointestinal pain control (C. Rousseaux et al., 2007).32
Lipidome: The lipidome is a full accounting of all lipids embedded in the membranes of each cell. Polyunsaturated fatty acids (PUFAs) are their essential building blocks. Since the human body does not make PUFAs, we must consume them to survive and thrive. PUFAs, such as omega-3 and omega-6 fatty acids, are the body’s primary building blocks for making its endocannabinoids. And here is an important insight: a healthy ECS is dependent on the ratio of omega-3 to omega-6. Omega-3s are considered anti-inflammatory, while omega-6 is a precursor to several potent pro-inflammatory mediators. Unfortunately, our modern western diet is heavy in omega-6 and low in omega-3 at an estimated ratio of 20:1 (respectively). Research suggests a healthy balance is 5:1 (omega-6:omega-3) (C. Bosch-Bouju et al., 2015).33
Early research trends suggest a broad therapeutic role for the crosstalk between the ECS and the lipidome. More specifically, mice fed omega-3s and CBD live longer than their control group (I. Abi et al., 2022).34 In humans, omega-3s (DHA and EPA) balance endocannabinoid overactivity with relevance to patients challenged with metabolic syndrome, obesity, and diabetes (K. Berge et al., 2013).35
Foods and oils rich in omega-3 include fish such as salmon, mackerel, and sardines; cod liver oil, hemp seed oil, flax seed oil, and chia seed oil are good sources (as are the seeds themselves).
Food and Nutrition: Research into modulators of the eCBome has highlighted several food choices we can make to support a healthy ECS. In general, a keto diet has been discovered to enhance CB1 and CB2 receptor site expression (I. Gigante et al., 2021).36 more precisely, one of the more studied food items, i.e., curcumin, one of turmeric’s most biologically active ingredients, has been shown to interact with the ECS.
Curcumin influences the expression of CB1 and CB2 and thus induces a sustained and dose-dependent modulation of endocannabinoid signaling, which in turn may be utilized to generate several clinically relevant effects. For example, recent research insights have found that curcumin is an agonist at CB2 (H. Pawar et al., 2022)37 with potential relevance to many chronic patient populations suffering from a shared underlying pathology of inflammation and oxidative stress. Furthermore, research trends suggest that curcumin may be an antagonist at CB1 (Z. Zhang et al., 2013).38 If confirmed in humans, it may be relevant in treating metabolic syndrome, obesity, and type 2 diabetes with THCV (a cannabis constituent) or similar CB1 antagonists.
In conclusion, one of the more exciting aspects of working with modulators of the eCBome is that, except for cannabis-based products, government policies, and control support all other pathways or mechanisms that allow us to harness the eCBome in the clinical setting. They are easy to obtain almost everywhere without a prescription, fun to learn about, and can be used to meet specific needs or preferences regarding establishing one’s optimal healthcare regimen.
Endnotes:
1. Benson H, Greenwood MM, Klemchuk H. The relaxation response: psychophysiologic aspects and clinical applications. Int J Psychiatry Med. 1975;6(1-2):87-98.
2. CannaKeys.com. Retrieved Jan. 26, 2023. https://cannakeys.com
3. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988 Nov). Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 34(5):605-613.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561-564.
Munro S, Thomas KL, Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65.
4. Irving, A.J., McDonald, N.A., Harkany, T. (2008). CB1 Cannabinoid Receptors: Molecular Biology, Second Messenger Coupling and Polarized Trafficking in Neurons. In: Köfalvi, A. (eds) Cannabinoids and the Brain. Springer, Boston, MA.
5. Zheng, G., Hong, S., Hayes, J. M., & Wiley, J. W. (2015). Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways. Experimental neurology, 273, 301–311.
Linton SJ. (2000 May) A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976). 25(9):1148-56.
Hall AM, Kamper SJ, Maher CG, Latimer J, Ferreira ML, Nicholas MK. (2011 May) Symptoms of depression and stress mediate the effect of pain on disability. Pain. 152(5):1044-1051.
Johnson, A. C., & Greenwood-Van Meerveld, B. (2014). Stress-induced pain: a target for the development of novel therapeutics. The Journal of pharmacology and experimental therapeutics, 351(2), 327–335.
6. Reiner K, Granot M, Soffer E, Lipsitz JD. (2016 Apr) A Brief Mindfulness Meditation Training Increases Pain Threshold and Accelerates Modulation of Response to Tonic Pain in an Experimental Study. Pain Med. 17(4):628-35.
7. Murillo-Rodríguez E, Arankowsky-Sandoval G, Rocha NB, Peniche-Amante R, Veras AB, Machado S, Budde H. (2018 Aug). Systemic Injections of Cannabidiol Enhance Acetylcholine Levels from Basal Forebrain in Rats. Neurochem Res. 43(8):1511-1518.
8. Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006 Feb;372(5):354-61.
9. Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids, and the nervous system. (2010 Aug) Immunobiology. 215(8):606-10.
10. Zádor F, Joca S, Nagy-Grócz G, Dvorácskó S, Szűcs E, Tömböly C, Benyhe S, Vécsei L. (2021 May) Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int J Mol Sci. 22(11):5903.
11. Terzian AL, Drago F, Wotjak CT, Micale V. (2011 Aug) The Dopamine and Cannabinoid Interaction in the Modulation of Emotions and Cognition: Assessing the Role of Cannabinoid CB1 Receptor in Neurons Expressing Dopamine D1 Receptors. Front Behav Neurosci. 5:49.
12. Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. (2006 Feb) Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 372(5):354-61.
13. Niederhoffer N, Hansen HH, Fernandez-Ruiz JJ, Szabo B. (2001 Nov) Effects of cannabinoids on adrenaline release from adrenal medullary cells. Br J Pharmacol. 134(6):1319-27.
14. Lee SH, Földy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. (2010 Jun) J Neurosci. 30(23):7993-8000.
15. Senin LL, Al-Massadi O, Folgueira C, Castelao C, Pardo M, Barja-Fernandez S, Roca-Rivada A, Amil M, Crujeiras AB, Garcia-Caballero T, Gabellieri E, Leis R, Dieguez C, Pagotto U, Casanueva FF, Seoane LM. (. 2013 Nov) The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One 8(11):e80339.
16. Patel KN, Joharapurkar AA, Patel V, Kshirsagar SG, Bahekar R, Srivastava BK, Jain MR. (2014 Dec) Cannabinoid receptor 1 antagonist treatment induces glucagon release and shows an additive therapeutic effect with GLP-1 agonist in diet-induced obese mice. Can J Physiol Pharmacol. 92(12):975-83.
17. Köfalvi A, Moreno E, Cordomí A, Cai NS, Fernández-Dueñas V, Ferreira SG, Guixà-González R, Sánchez-Soto M, Yano H, Casadó-Anguera V, Cunha RA, Sebastião AM, Ciruela F, Pardo L, Casadó V, Ferré S. (2020 Jan) Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol. 18(1):9.
18. Laguerre A, Keutler K, Hauke S, Schultz C. (2021 Jan) Regulation of Calcium Oscillations in β-Cells by Co-activated Cannabinoid Receptors. Cell Chem Biol. 28(1):88-96.e3.
19. Bosier B, Bellocchio L, Metna-Laurent M, Soria-Gomez E, Matias I, Hebert-Chatelain E, Cannich A, Maitre M, Leste-Lasserre T, Cardinal P, Mendizabal-Zubiaga J, Canduela MJ, Reguero L, Hermans E, Grandes P, Cota D, Marsicano G. Astroglial CB1 cannabinoid receptors regulate leptin signaling in mouse brain astrocytes. (2013 Aug) Mol Metab. 2(4):393-404.
20. Wyrofsky RR, Reyes BAS, Zhang XY, Bhatnagar S, Kirby LG, Van Bockstaele EJ. (2019 May) Endocannabinoids, stress signaling, and the locus coeruleus-norepinephrine system. Neurobiol Stress. 11:100176.
21. Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, Gall CM, Piomelli D. (2015 Nov) Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci U S A. 112(45):14084-9.
22. Ibarra-Lecue I, Diez-Alarcia R, Urigüen L. Serotonin 2A receptors and cannabinoids. (2021) Prog Brain Res. 259:135-175.
23. Lim J, Squire E, Jung KM. Phytocannabinoids, the Endocannabinoid System and Male Reproduction. (2023 Jan) World J Mens Health. 41(1):1-10.
24. Luce V, Fernandez Solari J, Rettori V, De Laurentiis A. The inhibitory effect of anandamide on oxytocin and vasopressin secretion from neurohypophysis is mediated by nitric oxide. (2014 Jan) Regul Pept. 188:31-9.
25. Ferrara, M. S. (2021). Peak-experience and the entheogenic use of cannabis in world religions, Journal of Psychedelic Studies, 4(3), 179-191.
26. Brugnatelli V, Facco E, Zanette G. (2021 Jul) Lifestyle Interventions Improving Cannabinoid Tone During COVID-19 Lockdowns May Enhance Compliance with Preventive Regulations and Decrease Psychophysical Health Complications. Front Psychiatry. 12:565633.
27. Sadhasivam S, Alankar S, Maturi R, Vishnubhotla RV, Mudigonda M, Pawale D, Narayanan S, Hariri S, Ram C, Chang T, Renschler J, Eckert G, Subramaniam B. (2020 Jun) Inner Engineering Practices and Advanced 4-day Isha Yoga Retreat Are Associated with Cannabimimetic Effects with Increased Endocannabinoids and Short-Term and Sustained Improvement in Mental Health: A Prospective Observational Study of Meditators. Evid Based Complement Alternat Med. 2020:8438272.
28. de Melo Reis RA, Isaac AR, Freitas HR, de Almeida MM, Schuck PF, Ferreira GC, Andrade-da-Costa BLDS, Trevenzoli IH. (2021 Oct) Quality of Life and a Surveillant Endocannabinoid System. Front Neurosci. 15:747229.
29. Johnstad PG. Cannabis as entheogen: survey and interview data on the spiritual use of cannabis. (2020 Sep) J Cannabis Res. 2(1):30.
30. Srivastava RK, Lutz B, Ruiz de Azua I. (2022 May) The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front Cell Neurosci. 16:867267.
31. Brugnatelli V, Facco E, Zanette G. (2021 Jul) Lifestyle Interventions Improving Cannabinoid Tone During COVID-19 Lockdowns May Enhance Compliance with Preventive Regulations and Decrease Psychophysical Health Complications. Front Psychiatry. 12:565633.
32. Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. (2007 Jan) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 13(1):35-7.
33. Bosch-Bouju C and Layé S (2016) Dietary Omega-6/Omega-3 and Endocannabinoids: Implications for Brain Health and Diseases. Cannabinoids in Health and Disease. InTech.
34. Abi, I., Yongu, A., Tersoo, U., Adeniyi, O. and Saalu, L. (2022) Omega 3 Fatty Acid and Cannabidiol Prolong Lifespan and Ameliorates Brain Ischaemia in Mice Fed Chronic High Fat Diet. Journal of Behavioral and Brain Science, 12, 335-341.
35. Berge K, Piscitelli F, Hoem N, Silvestri C, Meyer I, Banni S, Di Marzo V. (2013 May) Chronic treatment with krill powder reduces plasma triglyceride and anandamide levels in mildly obese men. Lipids Health Dis. 12:78.
36. Gigante I, Tutino V, Russo F, De Nunzio V, Coletta S, Armentano R, Crovace A, Caruso MG, Orlando A, Notarnicola M. (2021 Mar) Cannabinoid Receptors Overexpression in a Rat Model of Irritable Bowel Syndrome (IBS) after Treatment with a Ketogenic Diet. Int J Mol Sci. 22(6):2880.
37. Pawar HD, Mahajan UB, Nakhate KT, Agrawal YO, Patil CR, Meeran MFN, Sharma C, Ojha S, Goyal SN. (2022 Apr) Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life (Basel). 12(5):624.
38. Zhang Z, Guo Y, Zhang S, Zhang Y, Wang Y, Ni W, Kong D, Chen W, Zheng S. (2013 Dec) Curcumin modulates cannabinoid receptors in liver fibrosis in vivo and inhibits extracellular matrix expression in hepatic stellate cells by suppressing cannabinoid receptor type-1 in vitro. Eur J Pharmacol. 721(1-3):133-40.