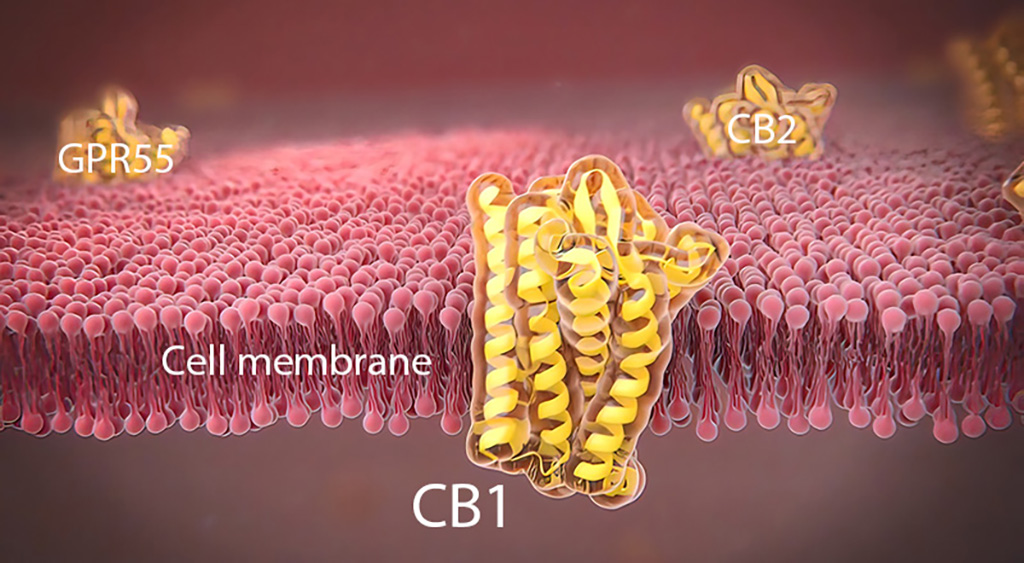
The History of CB1
The endocannabinoid receptor 1 (CB1) was discovered and characterized by a research team at the Department of Pharmacology, St. Louis University Medical School, Missouri (W. Devane et al., 1988).1
Subsequently, CB1 was cloned by a team from the Laboratory of Cell Biology, National Institutes of Mental Health, Bethesda, Maryland (L. Matsuda et al., 1990).2
Another team from the Department of Pharmacology, Meharry Medical College, Nashville, TN 37208, USA, cloned and sequenced the complementary DNA (cDNA) of CB1 using a mouse brain sample (A Chakrabarti et al., 1995).3
CB1 Gene
The official name for the gene that codes for CB1 is CNR1 (National Library of Medicine, 2023).4
CB1 is a guanine-nucleotide-binding protein (G-protein) coupled receptor family member, which inhibits adenylate cyclase activity in a dose-dependent, stereoselective, and pertussis toxin-sensitive manner (National Library of Medicine, 2023).4
CB1 General Expression and Distribution
CB1 is considered the most abundant GPR in the brain (A. Irving et al., 2008).5
CB1 is highly expressed in neurons of the central nervous system (CNS). It is found in excitatory and inhibitory neurons as well as in astrocytes. As such, CB1 has the potential to affect a diverse group of disease states associated with CNS malfunctions.
On the downside, CB1 density in the brain is also responsible for several of the adverse effects associated with CB1 agonists such as THC for instance.
However, novel research looking at peripheral CB1, biased CB1, or isolating allosteric (indirect) CB1 signaling suggests a novel strategy that may allow us to harness some of the CB1-induced effects while avoiding others.
CB1 is expressed predominantly, but not exclusively, in the central nervous systems (i.e., the brain and spinal cord) but also, albeit to a lesser degree, in organs and tissues of adrenal glands, heart, lung, prostate, uterus, ovary, testis, bone marrow, thymus, and tonsils for example (S. Galiègue et al., 1995).6
CB1 Distribution in the Human Brain
Very high expressions of CB1 were found in the dentate gyrus, Ammons’s horn, and subiculum of the hippocampal formation (M. Glass et al., 1997).7
High concentrations of CB1 were present in the entorhinal cortex and amygdaloid complex (M. Glass et al., 1997).7
CB1 was present in all regions of the neocortex. The highest concentrations of CB1 were found in the associational cortical regions of the frontal and limbic lobes. Moderate CB1 densities were present in the secondary sensory and motor cortical regions. The lowest CB1 expression was found in the primary sensory and motor cortical regions (M. Glass et al., 1997).7
In the midbrain, some of the highest densities of CB1 in the human brain were present in the substantia nigra pars reticulata (M. Glass et al., 1997).7
The highest amounts of CB1 in the hindbrain were found in the cerebellar cortex’s molecular layer and the vagus’s dorsal motor nucleus, with moderate densities of receptors in the nucleus of the solitary tract (M. Glass et al., 1997).7
The spinal cord showed low levels of CB1 expression (M. Glass et al., 1997).7
CB1’s Interaction with Neurotransmitters, Hormones, and Other Communication Molecules
CB1 primarily expresses on presynaptic terminals and functions to reduce the release of excitatory and inhibitory neurotransmitters such as acetylcholine, noradrenaline, serotonin, GABA, glutamate, and dopamine for example (B. Szabo et al., 2005).8
CB1 deficiency alters normal progesterone and estrogen levels, which has been associated with preterm birth in mice (H. Wang et al., 2008).9
CB1 and the Mitochondria
CB1 is expressed in the membrane of the mitochondria, which is associated with improved mitochondrial function. More specifically, regulation of neuronal energy metabolism and mitochondrial respiration(G. Bénard et al. 2012; J. Mendizabal-Zubiaga et al. 2016).10
CB1 Polymorphism
The term polymorphism is derived from the Greek polús- “many” and -morphḗ “shape,” which put together means many forms. As such, polymorphism refers to a common variation (not caused by a mutation) in a specific section of the DNA of an individual or in a group that can either induce a beneficial, harmful, or neutral effect on the organism. For instance, genetic studies showed mixed results when associating polymorphisms in the CNR1 gene with an increased risk of developing schizophrenia (I. Chavarría-Siles et al., 2008; J. Seifert et al., 2007).11
Diminished Expression of CB1 is Associated With:
Depression: Mice without CB1 are depressed ().12
Aging: Mice without CB1 age rapidly (brain and skin)()13 especially in diabetics ().14
Diminished capacity for healing: Mice bred without CB1 heal slower ().15
Neurological deficits: Mice without CB1 develop epilepsy ().16
Variables that Determine CB1 Expression
Aging in humans reduces CB1 sites by ~50% ().17
Humans consuming high-cholesterol diets express fewer CB1 sites ().18
Curcumin decreases CB1 expression (Z. Zhang et al., 2012).19
CB1 is a potential multimodal target of curcumin (T. Ramaholimihaso et al., 2020;20 P. Hassanzadeh et al., 2012).21
Physical exercise increased the expression and activation of CB1 (G. Galdino et al., 2014).22
CB1 Agonism vs Antagonism
CB1 agonism and CB1 antagonism tend to induce opposing effects. For instance, THC is an agonist at CB1 and can induce a dose-dependent euphoria while THCV and antagonist at CB1 can induce a dose-dependent dysphoria.
More specifically, you may have noticed various terms in the scientific literature i.e., agonism, antagonism, inverse agonism, or positive or negative allosteric (indirect) modulator. You may find the following discernment between the terms instructive.
Agonist: Interacts with a receptor site to produce a specific effect.
Antagonist: Interacts to block or reduce the normal effect.
Inverse Agonist: Interacts to create the opposite effect.
Positive or Negative Allosteric (indirect) Modulator: Allosteric binding sites are indirect binding sites (at or around a receptor) that can influence the effect of an agonist or antagonist.
Endnotes:
1. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. (1988 Nov) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 34(5):605-13.
2. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990 Aug) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 346(6284):561-4.
3. Chakrabarti A, Onaivi ES, Chaudhuri G. (1995) Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq. 5(6):385-8.
4. National Library of Medicine. Retrieved January 27, 2023. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1268
5. Irving, A.J., McDonald, N.A., Harkany, T. (2008). CB1 Cannabinoid Receptors: Molecular Biology, Second Messenger Coupling and Polarized Trafficking in Neurons. In: Köfalvi, A. (eds) Cannabinoids, and the Brain. Springer, Boston, MA.
6. Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. (1995 Aug). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 232(1):54-61.
7. Glass M, Dragunow M, Faull RL. (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 77(2):299-318.
8. Szabo, B. & Schlicker, E. (2005). Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 168, 327–365.
9. Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS One. 2008 Oct 3;3(10):e3320.
10. Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutiérrez S, Martín-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, López-Rodríguez ML, Grandes P, Rossignol R, Marsicano G. (2012 Mar 4). Mitochondrial CB₁ receptors regulate neuronal energy metabolism. Nat Neurosci. 15(4):558-64.
Mendizabal-Zubiaga J, Melser S, Bénard G, Ramos A, Reguero L, Arrabal S, Elezgarai I, Gerrikagoitia I, Suarez J, Rodríguez De Fonseca F, Puente N, Marsicano G, Grandes P. (2016 Oct 25) Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front Physiol. 7:476.
11. Chavarría-Siles, I. et al. Cannabinoid receptor 1 gene (CNR1) and susceptibility to a quantitative phenotype for hebephrenic schizophrenia. (2008) Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 147, 279–284.
Seifert J, Ossege S, Emrich HM, Schneider U, Stuhrmann M. (2007 Oct). No association of CNR1 gene variations with susceptibility to schizophrenia. Neurosci Lett. 426(1):29-33.
12. Valverde O, Torrens M. (2012 Mar) CB1 receptor-deficient mice as a model for depression. Neuroscience. 204:193-206.
13. Bilkei-Gorzo A, Drews E, Albayram Ö, Piyanova A, Gaffal E, Tueting T, Michel K, Mauer D, Maier W, Zimmer A. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1⁻/⁻ mice. Neurobiol Aging. 2012 Jan;33(1):200.e11-22.
14. C Leal E, F Moura LI, Pirzgalska RM, Marques-da-Silva D, Ledent C, Köfalvi A, Carvalho E. (2021 Oct) Diabetes and Cannabinoid CB1 receptor deficiency promote similar early onset aging-like changes in the skin. Exp Gerontol. 154:111528.
15. Ruhl T, Lippold EF, Christer T, Schaefer B, Kim BS, Beier JP. (2021 Nov) Genetic deletion of the cannabinoid receptors CB1 and CB2 enhances inflammation with diverging effects on skin wound healing in mice. Life Sci. 285:120018.
16. Rowley S, Sun X, Lima IV, Tavenier A, de Oliveira ACP, Dey SK, Danzer SC. Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia. 2017 Dec;58(12):e162-e166. 6. Carrera, J., Tomberlin, J., Kurtz, J., Karakaya, E., Bostanciklioglu, M., & Albayram, O. (2021).
17. Kataoka K, Bilkei-Gorzo A, Nozaki C, Togo A, Nakamura K, Ohta K, Zimmer A, Asahi T. (2020 Jul) Age-dependent Alteration in Mitochondrial Dynamics and Autophagy in Hippocampal Neuron of Cannabinoid CB1 Receptor-deficient Mice. Brain Res Bull. 160:40-49.
18. Hayakawa K, Mishima K, Nozako M, Hazekawa M, Aoyama Y, Ogata A, Harada K, Fujioka M, Abe K, Egashira N, Iwasaki K, Fujiwara M. (2007 Mar) High-cholesterol feeding aggravates cerebral infarction via decreasing the CB1 receptor. Neurosci Lett. 414(2):183-7.
19. Zhang Z, Guo Y, Zhang S, Zhang Y, Wang Y, Ni W, Kong D, Chen W, Zheng S. Curcumin modulates cannabinoid receptors in liver fibrosis in vivo and inhibits extracellular matrix expression in hepatic stellate cells by suppressing cannabinoid receptor type-1 in vitro. Eur J Pharmacol. 2013 Dec 5;721(1-3):133-40.
20. Ramaholimihaso T, Bouazzaoui F, Kaladjian A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front Psychiatry. 2020 Nov 27;11:572533.
21. Hassanzadeh P, Hassanzadeh A. The CB₁ receptor-mediated endocannabinoid signaling and NGF: the novel targets of curcumin. Neurochem Res. 2012 May;37(5):1112-20.
22. Galdino G, Romero T, Pinho da Silva JF, Aguiar D, de Paula AM, Cruz J, Parrella C, Piscitelli F, Duarte I, Di Marzo V, Perez A. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg. 2014 Sep;119(3):702-715.

