Surprising Benefits from an Unexpected Source
Have you ever wondered what contributes to nature’s abundance of colors and scents that makes being around lush, aromatic plants so delightful? By the same token do you know what natural ingredients contribute to making one of the most basic pleasure of life, the taste of foods, so enjoyable? The answer to both is Flavonoids. Also known as bioflavonoids these natural substances are produced by plants such as citrus fruits, berries, vegetables, tea, cocoa, red wine and cannabis.
Flavonoids are a biologically diverse family of natural substances that help realize a number of beneficial effects to the plants that make them and those who consume them. Named after the Latin word for yellow (flavus) presumably chosen to describe the compounds that influence color schemes in plants but also help to generate their unique flavors and scents. Flavonoids are considered secondary metabolites that function to facilitate biological interactions within the same organism, between other organisms, and the environment. As such, flavonoids are not primary life-sustaining building blocks like sugars, proteins, or lipids but rather plant constituents that realize secondary benefits. In plants they function to attract pollinators, detract pests, protect against shifts in salinity, safeguard against UV light or establish freezing tolerances.
Chronic Conditions and Flavonoids
In people (or any other mammal) who consume them, flavonoids can mitigate some of the shared underlying pathologies of numerous chronic conditions and in doing so increase an organisms ability to generate and maintain health and wellness. More specifically, flavonoids help to make the process of managing chronic internal and environmental stressors such as the aging process,1 oxidative stress or inflammation,2 more efficient. Furthermore, if we take into consideration flavonoid’s very low adverse effects potential and its potential protective effects against environmental threats such as various viral diseases including COVID,3 for example it becomes easy to understand why flavonoids are found in a great number of pharmaceutical, nutraceutical, as well as cosmetic products generated across the globe. Indeed, most every Health Agency tasked with educating people about dietary health recommend fruits and vegetables abundant in flavonoids for these very reasons. However, due to their relatively low bioavailability, rapid metabolism, and elimination most people could benefit from an increase intake of flavonoids.
Chemical Structure of Flavonoids
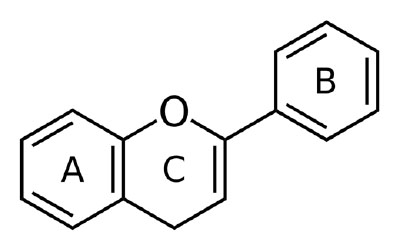
Chemically, flavonoids present with variable phenolic structures (organic aromatic building blocks) that include thousands of individual members. Those that are of dietary importance are typically divided into six major sub-groups (in alphabetical order with food examples): anthocyanidins (blue berries, wine), flavan-3-ols (green tea, cocoa, various spices), flavanones (fresh lemon, fresh oranges), flavones (fresh parsley, fresh thyme, cannabis), flavonols (tea, fresh, raw kale, cannabis), and isoflavones (mature raw soybean seeds, firm, cooked tofu).4 For those readers interested in a bit more technical information flavonoids have a typical molecular structure containing a 15-carbon structure (abr. C6-C3-C6) comprised of three rings (i.e. two phenyl rings each made up of six carbon atoms (A, B) and one heterocyclic ring characterized by 2 atoms of at least 2 elements in this case carbon and oxygen (C).
Cannaflavins
Cannabis-based flavonoids primarily belong two sub-classes i.e., flavones and flavonols. The earliest paper describing three cannabis-based flavonoids was published by Canadian researchers in 1979.5 By 2021 a team from the US described a total of 34 flavonoids (for a complete list in order of discovery scroll down to the end of article).6
And, while the scientific literature reporting on cannaflavins in the context of health, healing and well-being is relatively small, emerging data is beginning to describe cannflavin-induced effects with potential clinical relevance most notably anti-inflammatory,7 anti-oxidant8 and potential analgesic effects.9 Specific treatment examples include the potential mitigation of neurodegenerative processes with potential relevance to patients with Alzheimer’s disease.10 Also of note, the unnatural isomer of Cannflavin B (i.e. FBL-03G) has demonstrated therapeutic potential in preclinical models of metastatic pancreatic cancer notorious for extremely poor survival rates and orthodox treatment responses.11
Consider these emerging facts with potential practical relevance:
- Flavonoids are not present in cannabis roots, stem or barks.12
- Flavonoids are present in smaller amounts in cannabis flower (i.e., 0.07–0.14%).13
- Flavonoids occurred in the highest concentrations in the leaves of cannabis (0.34–0.44%).14
- Total flavonoid content in cannabis flowers was significantly higher in a cannabis chemotype III than Chemotype I and Chemotype II.15
- Total flavonoid content in leaves was higher in Chemotype II and Chemotype III than in Chemotype I.16
- Flavonoid content drops as cannabis ages.17
- Sprouting hemp seeds (rich in Omega-3 Fatty Acid) induces the production of cannflavins A and B.18
The Takeaway: Cannabis keeps on surprising us. The evidence-based underpinnings of flavonoids are but one example. Scientists continuously discover novel plant constituents, novel therapeutic effects, and novel complexities, which taken together allows us to make more informed and discerning decisions to amplify the health and wellness generating effects of cannabis-based therapeutics.
When exploring flavonoid profiles consider choosing a cannabis chemotype III (more CBD than THC), consider leaf-based cannabis products (e.g., leaf-based shake/kief), sprout hemp seeds, and/or juice fresh cannabis leaves. Alternatively, to maximize flavonoid content you can utilize fresh cannabis flower. The cannabinoids in fresh flower have not yet decarboxylated (i.e., do not produce cognitive changes) and as such exist in their acid forms with relatively high amounts of flavonoids and terpenes. If the taste is too unpleasant due its bitterness you may want to mix them with carrot juice or chop them up and fill a few gelatin capsules for easier consumption.
Cannabis-Based Flavonoids by Year of Discovery (Radwan et. al. 2021):19
1979 (Clark M.N. and Bohm B.A.):20 Vitexin • cytisoside • cytisoside glucoside
1980 (Turner CE, Elsohly MA, Boeren EG):21
Orientin • orientin-O-glucoside • orientin-7-O-glucoside • orientin-7-O-rhamnoglucoside ••
Vitexin-O-glucoside • vitexin-7-O-glucoside • vitexin-7-O- rhamnoglucoside ••
Isovitexin • isovitexin-O-glucoside • isovitexin-7-O-glucoarbinoside • isovitexin-7-O- rhamnoglucoside • apigenin-7-O-glucoside • apigenin-7-O-glucoronoid • apigenin-7-O-p-coumaroylglucoside ••
Luteolin-C-glucuronid • luteolin-7-0-glucuronid • kaempferol-3-0-diglucoside • quercetin-3-0-glucoside • quercetin-3-0-diglucoside
1982 (Crombie L. and Crombie W.M.L):22 Canniflavone 1 • Canniflavone 2
1986 (Barrett ML, Scutt AM, and Evans FJ):23 Cannflavin A • Cannflavin B (same compounds as above)
2005 (Ross SA, ElSohly MA. et. al.):24 Kaempferol-3-O-sophoroside • quercetin-3-O-sophoroside
2008 (Radwan et. al.):25 Cannflavin C • 6-prenylapigenin • chrysoeriol
2008 (Cheng L., Kong D., and Hu G.):26 Apigenin-6,8-di-C-β-D-glucopyranoside
2012 (Chen B., Cai G. et. al.):27 Rutin
2020 (Ingallina C. et. al.):28 Quercetin • Naringenin • Naringin
Endnotes Flavonoids in Cannabis:
1. Domaszewska-Szostek A, Puzianowska-Kuźnicka M, Kuryłowicz A. (2021 Jun 25) Flavonoids in Skin Senescence Prevention and Treatment. Int J Mol Sci. 22(13):6814.
Celińska A. (2021 Jun 9) Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int J Mol Sci. 22(12):6219.
2. Arcusa R, Carrillo JÁ, Xandri-Martínez R, Cerdá B, Villaño D, Marhuenda J, Zafrilla MP. (2021 Jun 12) Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Molecules. 26(12):3604.
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. F2020 Feb 12) Flavonoids as Anticancer Agents. Nutrients. 12(2):457.
Hazafa A, Rehman KU, Jahan N, Jabeen Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr Cancer. 2020;72(3):386-397.
3. Mhatre S, Srivastava T, Naik S, Patravale V. (2021 May) Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 85:153286.
Ohgitani E, Shin-Ya M, Ichitani M, Kobayashi M, Takihara T, Kawamoto M, Kinugasa H, Mazda O. (2021) Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules. 26(12):3572.
Wang YQ, Li QS, Zheng XQ, Lu JL, Liang YR. (2021 Jun 29) Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules. 26(13):3962.
Chowdhury P, Sahuc ME, Rouillé Y, Rivière C, Bonneau N, Vandeputte A, Brodin P, Goswami M, Bandyopadhyay T, Dubuisson J, Séron K. (2018 Nov 28) Theaflavins, polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture. PLoS One. 13(11).
Godinho PIC, Soengas RG, Silva VLM. (2021 Jun 7) Therapeutic Potential of Glycosyl Flavonoids as Anti-Coronaviral Agents. Pharmaceuticals (Basel). 14(6):546.
4. Sebastian, R. S., Wilkinson Enns, C., Goldman, J. D., Martin, C. L., Steinfeldt, L. C., Murayi, T., & Moshfegh, A. J. (2015). A New Database Facilitates Characterization of Flavonoid Intake, Sources, and Positive Associations with Diet Quality among US Adults. The Journal of nutrition, 145(6), 1239–1248.
5. Clark M.N., Bohm B.A. (1979) Flavonoid variation in Cannabis. Bot. J. Linn. Soc. 79:249–257.
6. Radwan, M. M., Chandra, S., Gul, S., & ElSohly, M. A. (2021). Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules (Basel, Switzerland), 26(9), 2774.
7. Erridge S, Mangal N, Salazar O, Pacchetti B, Sodergren MH. (2020 Oct) Cannflavins – From plant to patient: A scoping review. Fitoterapia. 146:104712.
Rea KA, Casaretto JA, Al-Abdul-Wahid MS, Sukumaran A, Geddes-McAlister J, Rothstein SJ, Akhtar TA. (2019 Aug) Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry. 164:162-171.
8. André, A., Leupin, M., Kneubühl, M., Pedan, V., & Chetschik, I. (2020). Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis Sativa L. Cultivated at Three Sowing Densities. Plants (Basel, Switzerland), 9(12), 1740.
Rea KA, Casaretto JA, Al-Abdul-Wahid MS, Sukumaran A, Geddes-McAlister J, Rothstein SJ, Akhtar TA. (2019 Aug) Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry. 164:162-171.
9. Rodriguez CEB, Ouyang L, Kandasamy R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav Pharmacol. 2021 Mar 9. doi: 10.1097/FBP.0000000000000627.
10. Eggers C, Fujitani M, Kato R, Smid S. (2019 Nov) Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid β-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone. Biochem Pharmacol. 169:113609.
11. Moreau, M., Ibeh, U., Decosmo, K., Bih, N., Yasmin-Karim, S., Toyang, N., Lowe, H., & Ngwa, W. (2019). Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Frontiers in oncology, 9, 660.
12. Jin, D., Dai, K., Xie, Z., & Chen, J. (2020). Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Scientific reports, 10(1), 3309.
13. Ibid.
14. Ibid.
15. Ibid.
16. Ibid.
17. Ibid.
18. Werz, Oliver; Seegers, Julia; Schaible, Anja Maria; Weinigel, Christina; Barz, Dagmar; Koeberle, Andreas; Allegrone, Gianna; Pollastro, Federica; Zampieri, Lorenzo; Grassi, Gianpaolo; Appendino, Giovanni (2014). “Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase”. Pharmanutrition. 2 (3): 53–60.
19. Radwan, M. M., Chandra, S., Gul, S., & ElSohly, M. A. (2021). Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules (Basel, Switzerland), 26(9), 2774.
20. Clark M.N., Bohm B.A. (1979) Flavonoid variation in Cannabis. Bot. J. Linn. Soc. 79:249–257.
21. Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980 Mar-Apr;43(2):169-234.
22. Crombie L. and Crombie W.M.L. Natural products of Thailand high Δ 1-THC-strain Cannabis. The bibenzyl-spiran-dihydrophenanthrene group: Relations with cannabinoids and canniflavones. J. Chem. Soc. Perkin Trans. 1982;1:1455–1466.
23. Barrett, M. L.; Scutt, A. M.; Evans, F. J. (1986). “Cannflavin A and B, prenylated flavones from Cannabis sativa L”. Experientia. 42 (4): 452–453.
24. Ross SA, ElSohly MA, Sultana GN, Mehmedic Z, Hossain CF, Chandra S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem Anal. 2005 Jan-Feb;16(1):45-8.
25. Radwan, Mohamed M.; Elsohly, Mahmoud A.; Slade, Desmond; Ahmed, Safwat A.; Wilson, Lisa; El-Alfy, Abir T.; Khan, Ikhlas A.; Ross, Samir A. (2008). “Non-cannabinoid constituents from a high potency Cannabis sativa variety”. Phytochemistry. 69 (14): 2627–2633.
26. Cheng L., Kong D., Hu G., Hemp I. (2008) Chemical constituents from petroleum ether and n-butanol portions of methanol extract. Zhongguo Yiyao Gongye Zazhi. 39:18–21.
27. Chen B., Cai G., Yuan Y., Li T., He Q., He J. (2012) Chemical constituents in hemp pectin. Zhongguo Shiyan Fangjixue Zazhi. 18:98–100.
28. Ingallina, C., Sobolev, A. P., Circi, S., Spano, M., Fraschetti, C., Filippi, A., Di Sotto, A., Di Giacomo, S., Mazzoccanti, G., Gasparrini, F., Quaglio, D., Campiglia, E., Carradori, S., Locatelli, M., Vinci, G., Rapa, M., Ciano, S., Giusti, A. M., Botta, B., Ghirga, F., … Mannina, L. (2020). Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules (Basel, Switzerland), 25(8), 1908.